Patient specific instrumentation in total knee arthroplasty: a state of the art
Introduction
Patient specific instrumentation (PSI) is a modern technique in total knee arthroplasty (TKA) aiming to facilitate the implant of the prosthesis. The customized cutting blocks of the PSI is generated from pre-operative three-dimensional model, using computed tomography (CT) or magnetic resonance imaging (MRI) (1). A correct surgical plan is mandatory for a good surgical implant.
The success of a TKA depends on knee alignment, gap kinematics and soft tissue balancing, and all three depend on the proper position of the components.
The PSI guide takes into account any slight deformities or osteophytes and applies preoperative planning for bone resection, using the pre-determined implant size, position, and rotation. The apparent benefits of this technology are that neutral postoperative alignment is more reproducible, surgical time is decreased, and the entire procedure results more efficient and cost-effective. Many manufacturers have invested in PSIs (Table 1). Large debates have taken place about this topic during the last years and, at the moment, there is no consensus in literature regarding the accuracy and reliability of PSI as many studies have shown controversial and inconsistent results.

Full table
Preoperative planning and surgical technique
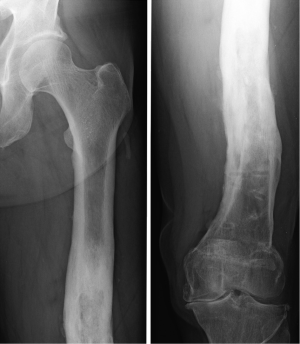
The use of PSI is indicated when advanced osteoarthritis, severe pain, and limited function/walking ability are present, such as in a standard instrumentation TKA. In addition to that, PSI finds its indication when intra-medullary guides cannot be used. For example, when there is a post-traumatic femoral deformity (Figure 1) (2).
PSI facilitates cutting guides by creating a 3-dimensional (3D) model of the knee preoperatively, using CT or MRI and a full-leg antero-posterior radiograph. With a specific software program manufacturing engineers turn 2D CT or MRI images into 3D representations of the knee and lower limb. Using these 3D images, the anatomical landmarks of the knee are easily identified. A preoperative planning with bony resections is then created and presented to the operating surgeon. Using a specific software, the operator is then able to evaluate the 3D planning of the TKA with the bony resections. During this phase, the surgeon is able to approve or modify the pre-operative plan, adjusting as needed the tibial and femoral bone resections. In this phase, it is also possible to accurately plan the depth and the coronal orientation of the resection, as well as the rotation and the slope of the cuts. The rotation of the femoral implant is based on the transepicondylar axis (3). The tibial rotation is controlled and set-up according to the anterior tibial tuberosity. After the operator’s authorization, custom cutting guides that fit on the patient’s anatomy are manufactured and then sent to the surgeon (Figures 2,3). The PSI femoral guides are used to determine the valgus angle, level of resection, alignment, rotation, and size of the femoral component, whereas the patient-specific tibial guides are used to determine tibial alignment, level of resection, and tibial slope and rotation. Usually, 3 to 4 weeks are required to the final production of these cutting guides (4).
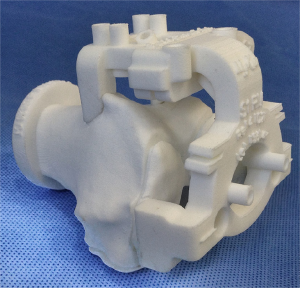
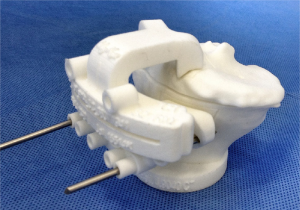
During surgery, the PSI guides are either used directly as slotted cutting guides or for an accurate pin positioning, using standard resection instrumentation for the bone cuts. The cutting guides are used for the primary distal femoral cut and proximal tibial cut. The subsequent bone cuts are achieved with standardized instrumentation. If the resections do not appear well aligned or orientated from the operator’s point of view, intraoperative modifications can be realized by using standard instrumentation for additional femoral and tibial cut. Using a MRI-based PSI, it is mandatory to leave cartilage, osteophytes and bone spurs as they act as a reference for cutting guide positioning. On the other hand, using a CT-based PSI, the cartilage and especially the soft tissues above the cutting blocks contact points must be accurately removed using electrocautery in order to properly expose the bone before pin fixation. These steps are compulsory, knowing that the CT-scan hardly detects cartilage or soft tissues during the planning. Without it, the CT-based PSI could be unstable and as a consequence, fail. The remaining procedure is then carried out as a usual TKA procedure.
Clinical results
Pre-operative planning and components alignment
In a comparison with navigation instrumentation, Conteduca et al. (5) demonstrated that PSIs are not fully able to achieve satisfactory alignment in both planes, especially evaluating the position of isolated tibial or femoral component. In a review by Sassoon et al. (6), the authors claim that there is a need to frequently change the cutting guides, modifying, as a result, the pre-operative planning. There is no complete consensus on the accuracy of the component alignment with a PSI. The importance of the coronal alignment in a TKA is well known. It should accurately match the mechanical axis and a higher complication rate has been reported if this result was not reached (7). Different authors showed how the coronal limb alignment as outliers in the frontal plane is an important factor in the implant survival, inducing an high risk of faster polyethylene wear (8,9).
With a traditional instrumentation, a series of cutting guides are used to provide bone resections in order to achieve correct alignment. With a PSI the final alignment results is checked after the preoperative planning using the appropriate software. But, as previously mentioned, the surgeon changes frequently, during surgery, the pre-operative planning in order to achieve better sizing and alignment. Rho et al. found no difference in alignment comparing PSI and traditional technique. But they reported a 16% rate of abandoned PSI guide due to not satisfactory alignment (10). They found an excessive femoral extra-rotation in 12% of cases. Similar results were observed by Stronach et al. (11), who reported an excessive femoral rotation in about 20% of cases. They also reported a decreased accuracy for tibial slope which results generally increased with the use of PSI instrumentation (38% PSI vs. 61% TI, P=0.01). Lustig et al. (12) comparing with the computer navigation, have evaluated the final alignment of the component, reporting that PSI do not improve accuracy. Also, Victor et al. (13) in 2014, have found more outliers in the sagittal and coronal alignment of the tibial component with the use of PSI in comparison to conventional instrumentation. Other authors, such as Barrett et al. (14) have tried to compare computer assisted surgery and PSI. A very small difference between the goup was found, with the computer assisted technique being slightly more accurate. Boonen et al. (15) have reported a 29% outliers from the mechanical axis, defined as exceeding a threshold of 3° of the mechanical axis of the lower limb in the coronal plane, using PSI instrumentation. Nunley et al. (16) reported a much higher rate, with 37% of patients recorded with malalignment.
However, despite these findings, some Authors report a greater accuracy with the PSI system, justifying it with a more precise preoperative planning that must nevertheless be carefully evaluated and not blindly accepted.
Anderl et al. (17) present results that show significantly superior accuracy in mechanical alignment restoration and 3D-component positioning compared with conventional instrumentation in primary TKA. In their prospective study, they detected a mean value of less than 2° deviations from targeted component position in all planes as well as in the hip-knee-ankle angle. In recent studies (18,19), Heyse et al. compared rotational component alignment using an MRI study following TKA. They report that PSI was effective in significantly reducing outliers of optimal rotational tibial component alignment and that in both PSI and conventional TKA, almost all outliers were in excessive external rotation, which may have less negative impact on the function of the TKA than internal rotation. Furthermore, they affirm that PSI technique improves the rotation of the femoral component in comparison with standard techniques, although the same mean rotational values are found in both groups.
In conclusion we have not found in literature, a universal consensus on the precision of the PSI instrumentation on obtaining a correct alignment of the components. From our experience, we are not used to blindly accept the planning proposed by the software, and we carefully check intraoperatively the correct alignment. By doing this, we have reduced the rate of outliers and inaccuracy of the component’s alignment.
Functional outcome
About the clinical and functional outcome, we have found in literature different results from the clinical studies. Yaffe et al. (20) report significantly greater improvement in functional score 6 months after surgery when compared to standard TKA. These results have to be carefully analyzed, considering that in this study, the Author have not performed a randomization of the patient’s groups. In fact, in the PSI group, the patients have higher preoperative knee score compared with the standard TKA group. On the other hand, different authors demonstrated recently, with high quality studies, that no significant clinical benefits could be demonstrated with personalized techniques. This has been established for both total and unicompartmental knee replacement (21-23).
Blood loss
Thienpont et al. (24) have evaluated the impact of PSIs on blood loss, comparing it with a conventional TKA. By the most, rod entry hole has been considered a source of peri and postoperative oozing resulting in an increased blood loss. PSI do not violate intramedullary canal mainly because the use of intramedullary rod is not necessary to set the correct alignment. Controversial data was found in literature on the use of extramedullary guide or navigation and blood loss (5,25-27).
In this study, however, it is demonstrated that the use of PSIs does not reduce blood loss in TKA and that a well-performed conventional TKA with bone plugging of the femoral hole and extramedullary tibial alignment can be considered as a blood sparing surgery that reduces hidden blood loss. Voleti et al. have found similar results (28).
Surgical time
It is a common idea that PSIs could theoretically shorten operating time, especially for less experienced surgeons. In a randomized controlled trial, Hamilton et al. (29) demonstrated that PSIs do not shorten surgical time in comparison with traditional cutting blocks surgery. On the other hand, other authors showed different results. Nunley et al. (16) and Voleti et al. (28) found similar surgical times, Bali et al. (30) found shorter skin-to-skin time compared with traditional surgery. Certainly, to reduce surgical time, the surgeon has to spend time in the preoperative planning to achieve the desired alignment, and the learning curve for PSI technique has to be completed.
Economic costs and effectiveness
Some surgeons support PSI technique as a useful way to save surgical time, with consequent economical benefits. In our opinion, a real cost/benefits analysis should take into account the amount of surgical time saved that could really be used for other procedures. For example, saving five of surgical time of a procedure that normally lasts 1 hour, does not improve the work rate of a surgical theatre. The pre-operative imaging and the cutting blocks custom-crafting costs should be considered, as well as the time spent by the surgeon during the pre-operative planning evaluation, as it has to be carefully analyzed in order to not jeopardize the final result.
In literature, to our knowledge, an accurate and complete analysis of the effective costs does not exists; for this reason, we cannot support the idea that PSIs have inferior nor superior costs than traditional instrumentation.
Conclusions
Literature does not suggest PSI techniques as a gold standard in TKA, and therefore it cannot be recommended as a standard technique in standard, not complicated primary TKA. Moreover, literature does not underline any improvement in components alignment, surgical time, blood loss or functional outcomes.
Further studies are needed to evaluate precisely the economic impact and effectiveness of PSIs.
Nevertheless, we think that in some particular situation, a patient specific cutting guide could improve results, especially for less experienced surgeons.
Furthermore, nowadays, many patients who underwent TKA suffered a previous trauma. In case of deformities, like femoral or tibial fractures healed with a malalignment, preoperative planning may result difficult, and some intra-operative technical difficulties can occur, such as the use of intra-medullar rod. In these selected cases, PSIs may be very useful to avoid errors in alignment and planning.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ast MP, Nam D, Haas SB. Patient-specific instrumentation for total knee arthroplasty: a review. Orthop Clin North Am 2012;43:e17-22. [Crossref] [PubMed]
- Sharareh B, Schwarzkopf R. Review article: Patient-specific versus standard instrumentation for total knee arthroplasty. J Orthop Surg (Hong Kong) 2015;23:100-6. [PubMed]
- Parratte S, Blanc G, Boussemart T, et al. Rotation in total knee arthroplasty: no difference between patient-specific and conventional instrumentation. Knee Surg Sports Traumatol Arthrosc 2013;21:2213-9. [Crossref] [PubMed]
- Camarda L, D'Arienzo A, Morello S, et al. Patient-specific instrumentation for total knee arthroplasty: a literature review. Musculoskelet Surg 2015;99:11-8. [Crossref] [PubMed]
- Conteduca F, Massai F, Iorio R, et al. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. Int Orthop 2009;33:1609-13. [Crossref] [PubMed]
- Sassoon A, Nam D, Nunley R, et al. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res 2015;473:151-8. [Crossref] [PubMed]
- Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br 1991;73:709-14. [PubMed]
- Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty 2009;24:39-43. [Crossref] [PubMed]
- Bargren JH, Blaha JD, Freeman MA. Alignment in total knee arthroplasty. Correlated biomechanical and clinical observations. Clin Orthop Relat Res 1983.178-83. [PubMed]
- Roh YW, Kim TW, Lee S, et al. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res 2013;471:3988-95. [Crossref] [PubMed]
- Stronach BM, Pelt CE, Erickson JA, et al. Patient-specific instrumentation in total knee arthroplasty provides no improvement in component alignment. J Arthroplasty 2014;29:1705-8. [Crossref] [PubMed]
- Lustig S, Scholes CJ, Oussedik SI, et al. Unsatisfactory accuracy as determined by computer navigation of VISIONAIRE patient-specific instrumentation for total knee arthroplasty. J Arthroplasty 2013;28:469-73. [Crossref] [PubMed]
- Victor J, Dujardin J, Vandenneucker H, et al. Patient-specific guides do not improve accuracy in total knee arthroplasty: a prospective randomized controlled trial. Clin Orthop Relat Res 2014;472:263-71. [Crossref] [PubMed]
- Barrett W, Hoeffel D, Dalury D, et al. In-vivo alignment comparing patient specific instrumentation with both conventional and computer assisted surgery (CAS) instrumentation in total knee arthroplasty. J Arthroplasty 2014;29:343-7. [Crossref] [PubMed]
- Boonen B, Schotanus MG, Kort NP. Preliminary experience with the patient-specific templating total knee arthroplasty. Acta Orthop 2012;83:387-93. [Crossref] [PubMed]
- Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res 2012;470:889-94. [Crossref] [PubMed]
- Anderl W, Pauzenberger L, Kölblinger R, et al. Patient-specific instrumentation improved mechanical alignment, while early clinical outcome was comparable to conventional instrumentation in TKA. Knee Surg Sports Traumatol Arthrosc 2016;24:102-11. [Crossref] [PubMed]
- Heyse TJ, Tibesku CO. Improved tibial component rotation in TKA using patient-specific instrumentation. Arch Orthop Trauma Surg 2015;135:697-701. [Crossref] [PubMed]
- Heyse TJ, Tibesku CO. Improved femoral component rotation in TKA using patient-specific instrumentation. Knee 2014;21:268-71. [Crossref] [PubMed]
- Yaffe M, Luo M, Goyal N, et al. Clinical, functional, and radiographic outcomes following total knee arthroplasty with patient-specific instrumentation, computer-assisted surgery, and manual instrumentation: a short-term follow-up study. Int J Comput Assist Radiol Surg 2014;9:837-44. [Crossref] [PubMed]
- Zhu M, Chen JY, Chong HC, et al. Outcomes following total knee arthroplasty with CT-based patient-specific instrumentation. Knee Surg Sports Traumatol Arthrosc 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Nam D, Park A, Stambough JB, et al. The Mark Coventry Award: Custom Cutting Guides Do Not Improve Total Knee Arthroplasty Clinical Outcomes at 2 Years Followup. Clin Orthop Relat Res 2016;474:40-6. [Crossref] [PubMed]
- Ollivier M, Parratte S, Lunebourg A, et al. The John Insall Award: No Functional Benefit After Unicompartmental Knee Arthroplasty Performed With Patient-specific Instrumentation: A Randomized Trial. Clin Orthop Relat Res 2016;474:60-8. [Crossref] [PubMed]
- Thienpont E, Grosu I, Paternostre F, et al. The use of patient-specific instruments does not reduce blood loss during minimally invasive total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 2015;23:2055-60. [Crossref] [PubMed]
- Baldini A, Adravanti P. Less invasive TKA: extramedullary femoral reference without navigation. Clin Orthop Relat Res 2008;466:2694-700. [Crossref] [PubMed]
- McConnell J, Dillon J, Kinninmonth A, et al. Blood loss following total knee replacement is reduced when using computer-assisted versus standard methods. Acta Orthop Belg 2012;78:75-9. [PubMed]
- Ajwani SH, Jones M, Jarratt JW, et al. Computer assisted versus conventional total knee replacement: a comparison of tourniquet time, blood loss and length of stay. Knee 2012;19:606-10. [Crossref] [PubMed]
- Voleti PB, Hamula MJ, Baldwin KD, et al. Current data do not support routine use of patient-specific instrumentation in total knee arthroplasty. J Arthroplasty 2014;29:1709-12. [Crossref] [PubMed]
- Hamilton WG, Parks NL, Saxena A. Patient-specific instrumentation does not shorten surgical time: a prospective, randomized trial. J Arthroplasty 2013;28:96-100. [Crossref] [PubMed]
- Bali K, Walker P, Bruce W. Custom-fit total knee arthroplasty: our initial experience in 32 knees. J Arthroplasty 2012;27:1149-54. [Crossref] [PubMed]


