Improving our understanding of papillary renal cell carcinoma with integrative genomic analysis
Introduction
The past decade has been one of marked transformation in the management of renal cell carcinoma (RCC). The dawn of the targeted therapy era in 2006, with the introduction of sunitinib and sorafenib, fundamentally altered the management of advanced RCC (1). Numerous drugs were subsequently approved that target either the VEGF/VEGFR or mTOR pathways (2-9) (Table 1). Unfortunately, despite the rapid expansion of our therapeutic armamentarium, RCC continues to remain a major cause of cancer morbidity and mortality. In the United States, RCC was the seventh and tenth most common malignancy among men and women, respectively, with an estimated new 62,700 cases and 14,240 deaths in 2016 alone (11).
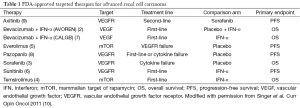
Full table
Papillary RCC (pRCC) accounts for approximately 15% of all kidney tumors making it the second most common histologic type of RCC (12). Until now, our understanding of the genetics and molecular biology of RCC has been primarily focused on clear cell RCC. As a result, there are no pRCC-specific FDA-approved therapies available to kidney cancer patients (13). However, in a recent publication in the New England Journal of Medicine, authors from The Cancer Genome Atlas (TCGA) Research Network describe a significant step towards understanding the molecular nature of pRCC (14). Identification of relevant molecular pathways and the accurate classification of pRCC subtypes are necessary to optimize clinical trial design and speed the development of novel targeted therapies.
Comprehensive molecular characterization of papillary renal cell carcinoma
Previously published next-generation sequencing studies have identified several mutated genes associated with pRCC including: MET, NF2, SETD2, and Nrf2 pathway genes (15,16). However, these mutations were found in only ~10–15% of pRCC tumors in these studies (15,16). The investigators of The Cancer Genome Atlas Research Network, in an attempt to improve our understanding and classification of pRCC, performed comprehensive molecular analysis, including whole-exome sequencing, identification of copy number alterations (CNAs), micro- and messenger-RNA sequencing, protein expression and DNA methylation analysis of 161 primary pRCC tumors (14).
Of these tumors, 75 were classified as papillary type 1 and 60 as type 2. As expected, the type 1 tumors were more likely to be lower grade than type 2 tumors. Analysis of CNAs resulted in the identification of three patterns: predominantly type 1 tumors with frequent gain of chromosomes 7 and 17; type 2 tumors with few CNAs; and type 2 tumors with aneuploidy, including frequent loss of chromosome 9p (14). Whole-exome sequencing identified 11 significantly mutated genes, including previously identified genes such as MET, SETD2, NF2 and BAP1, among others. These mutations, many of which are part of known cancer-associated pathways, were present in a higher percentage of tumors than was reported by previous studies.
The majority of type 1 pRCC tumors (81%) had gains of chromosome 7 or altered MET status (mutation, gene fusion or splice variant of MET) (14). While these findings support the hypothesis of MET as a driver mutation in type 1 pRCC, it cannot be concluded from this evidence alone. Further supporting this theory, however, is the finding that levels of MET mRNA expression were significantly higher in type 1 tumors than type 2 tumors (14).
CDKN2A alterations were found in 21 tumors (13%) and included 25% of type 2 tumors (14). These alterations included focal loss of 9p21, mutation, or promotor hypermethylation of CDKN2A (14). Additionally, increased expression of miR-10b-5p was correlated with decreased expression of CDKN2A (14). CDKN2A altered tumors were found, on univariate analysis, to be associated with lower overall survival when compared to tumors without CDKN2A alterations (14).
A novel CpG island methylator phenotype (CIMP) was identified in nine tumors, all of which also had hypermethylation of the CDKN2A promoter (14). Eight out of 9 of these tumors were papillary type 2. CIMP-associated tumors, like FH-deficient tumors in hereditary leiomyomatosis and renal cell cancer (HLRCC), were noted to have worse survival and gene expression changes consistent with a Warburg-like shift to glycolysis-dependent metabolism (17).
A cluster-of-clusters analysis was performed using the various data types to identify pRCC subgroups (14). Four subgroups were identified (C1, C2a, C2b, and C2c) and were associated with progressively worse overall survival. C1 included primarily papillary type 1 tumors, while C2a and C2b included primarily papillary type 2. Subgroup C2c included only type 2 pRCC with CIMP-associated tumors, which had the lowest overall survival (14).
This analysis, which elucidated the complexity of pRCC and the heterogeneity of type 2 pRCC specifically, has significant implications for the design of future clinical trials and the development of targeted therapies for pRCC.
Therapies for papillary renal cell carcinoma
While all the pivotal trials leading to the approval of targeted therapies for RCC have focused on clear cell histology thus far, recent studies have investigated the optimal treatment regimens in non-clear cell RCC. Sunitinib was tested in pRCC in the SUPAP trial and was found to be active in both type 1 (median OS 17.8 mo) and type 2 (median OS 12.4 mo) pRCC (18). The RAPTOR trial evaluated everolimus as monotherapy in pRCC and found that it was beneficial, with a median OS of 21 months and a similar difference between type 1 (median OS 28 mo) and type 2 (median OS 20 mo) (19). ASPEN (20) and ESPN (21) are two recently published phase 2 trials comparing sunitinib and everolimus as first line therapy in patients with metastatic non-clear cell RCC. Of note, there were significant differences in the trial populations—the ESPN trial included sarcomatoid clear cell RCC and 39.7% pRCC whereas ASPEN did not allow any clear cell RCC and 66% of subjects had pRCC. The ESPN trial was not able to show superiority of everolimus over sunitinib while the ASPEN trial concluded that sunitinib improved progression-free survival when compared to everolimus for non-clear cell RCC. Both trials, however, are limited by the significant heterogeneity of the non-clear cell RCC groups they studied and noted the need for improved patient stratification by molecular and genetic characteristics.
Foretinib, a multikinase inhibitor with activity against MET and VEGF receptors, among others, was evaluated in a phase 2 trial of patients with pRCC (22). Overall response was noted in 13.5% of subjects and median progression free survival (PFS) was 9.3 mo, with median OS not reached (22). Importantly, the presence of a germline MET mutation, but not other types of MET pathway activation, was predictive of response (22). In the TCGA analysis, only 3 of 75 type 1 pRCC tumors were found to have germline MET mutations, confirming this as a rare entity in sporadic cases (14). However, 81% of patients had some form of altered MET status and thus, MET remains a promising target in type 1 pRCC. Following this study of foretinib, multiple active trials are evaluating MET-inhibition in pRCC. One arm of EORTC 90101 (NCT01524926 “CREATE”) is evaluating crizotinib, an inhibitor of MET and ALK, in type 1 pRCC. Other small molecule MET inhibitors, INC280, tivantinib (ARQ-197), and AZD6094, are being investigated in active phase 2 trials (NCT02019693, NCT01688973, and NCT02127710, respectively).
An early prospective trial specific to pRCC evaluated single agent erlotinib, an EGFR tyrosine kinase inhibitor (TKI) (23). Erlotinib monotherapy had an overall response rate of 11% and was generally well tolerated in this trial (23). Combination therapy targeting VEGFR and EGFR using bevacizumab and erlotinib was shown to have activity in familial type 2 pRCC in a retrospective study of patients with hereditary leiomyomatosis and renal cell cancer (HLRCC) (24). Prospective evaluation of this approach is ongoing in a two-arm phase 2 trial (NCT01130519) enrolling patients with HLRCC as well as sporadic pRCC (25). Interim results from this study have demonstrated activity in both subsets of patients (26). Another ongoing phase I/II study (NCT02495103) is evaluating the multikinase inhibitor vandetanib (with activity against VEGFR, EGFR, and ABL1) in combination with metformin in patients with advanced HLRCC, succinate dehydrogenase (SDH) RCC, and sporadic pRCC. The TCGA analysis found that 11.2% of pRCC tumors had a loss of 1p36, which includes a negative regulator of EGFR and is associated with EGFR amplification (14). Patients with this anomaly may be more likely to benefit from EGFR-directed therapy with the addition of erlotinib to treatment regimens.
Future directions
The identification and characterization of the aberrant pathways and multiple subtypes of pRCC by the TCGA Research Network should lead to future trials that stratify subjects by the genetic characteristics of their tumors in order to identify and validate these potential prognostic and therapeutic biomarkers. For example, studies of EGFR-directed therapy may evaluate loss of 1p36 as a biomarker of improved response rates. Germline MET mutations have been associated with response to MET-directed TKI therapy (22) and future studies may evaluate the use of MET pathway activation as a predictive biomarker.
Nivolumab, a programmed death 1 (PD-1) checkpoint inhibitor, was approved in November 2015 by the FDA for second-line treatment of advanced clear cell RCC after a randomized phase III trial reported improved overall survival and fewer serious adverse events when compared to everolimus (22). Current and future research will evaluate checkpoint inhibition in neoadjuvant and adjuvant settings, as first-line therapy, and in combination therapy regimens for advanced disease. The use of checkpoint inhibition in pRCC is a promising area of investigation as 10% of pRCC tumors have been shown to express programmed death-ligand 1 (PD-L1) a rate similar to the expression in clear cell RCC tumors (27,28).
Conclusions
In this era of precision oncology, the ideal therapy is one that is targeted to the specific genetic and molecular abnormalities found in a patient’s tumor. TCGA Research Network investigators have made significant progress towards that goal in their elucidation of the aberrant pathways present in pRCC and in refining the categorization of papillary type 1 and 2 tumors. Papillary RCC is a heterogeneous disease and no systemic therapy has yet been recognized as the “gold standard” for patients with pRCC. With a better understanding of the underlying biology of this disease, future investigators will be able to identify more promising agents and design trials to include patients most likely to benefit from the proposed treatment. As the results of several pRCC trials have taught us, a one-size-fits-all strategy is not likely to result in good outcomes for our patients. Now, armed with information from this TCGA study, we can design smarter trials with better agents in order to find the best treatment for each patient every time.
Acknowledgements
Funding: This work is supported by a grant from the National Cancer Institute (P30CA072720).
Footnote
Provenance: This is a Guest Perspective commissioned by Guest Editor Xiongbing Zu, MD, PhD (Department of Urology, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Allard CB, Gelpi-Hammerschmidt F, Harshman LC, et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol Oncol 2015;33:496.e11-6.
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [Crossref] [PubMed]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [Crossref] [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24. [Crossref] [PubMed]
- Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol 2010;28:2137-43. [Crossref] [PubMed]
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061-8. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Singer EA, Gupta GN, Srinivasan R. Update on targeted therapies for clear cell renal cell carcinoma. Curr Opin Oncol 2011;23:283-9. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353:2477-90. [Crossref] [PubMed]
- Singer EA, Bratslavsky G, Linehan WM, et al. Targeted therapies for non-clear renal cell carcinoma. Target Oncol 2010;5:119-29. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016;374:135-45. [Crossref] [PubMed]
- Durinck S, Stawiski EW, Pavía-Jiménez A, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet 2015;47:13-21. [Crossref] [PubMed]
- Kovac M, Navas C, Horswell S, et al. Recurrent chromosomal gains and heterogeneous driver mutations characterise papillary renal cancer evolution. Nat Commun 2015;6:6336. [Crossref] [PubMed]
- Tong WH, Sourbier C, Kovtunovych G, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell 2011;20:315-27. [Crossref] [PubMed]
- Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)†. Ann Oncol 2015;26:1123-8. [Crossref] [PubMed]
- Escudier BJ, Bracarda S, Rey JP, et al. Open-label, phase II raptor study of everolimus (EVE) for papillary mRCC: Efficacy in type 1 and type 2 histology. J Clin Oncol 2014;32:abstr 410.
- Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol 2016;17:378-88. [Crossref] [PubMed]
- Tannir NM, Jonasch E, Albiges L, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol 2015. [Epub ahead of print]. [PubMed]
- Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol 2013;31:181-6. [Crossref] [PubMed]
- Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol 2009;27:5788-93. [Crossref] [PubMed]
- Singer EA, Marchalik D, Friend JC. Efficacy of combined vegf and egfr inhibtion in metastatic papillary rencal cell carcinoma associated with hereditary leiomyomatosis and renal cell cancer. J Canadian Urol 2012;19:6510.
- Singer EA, Friend JC, Hawks G, et al. A phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer (RCC). J Clin Oncol 2012;30:abstr TPS4680.
- Stamatakis L, Singer EA, Siddiqui MM, et al. Phase II trial of bevacizumab and erlotinib in patients with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell carcinoma. Eur J Cancer 2013;49:S659-S60.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol 2014;25:2178-84. [Crossref] [PubMed]


