Predominant histologic subtype in lung adenocarcinoma predicts benefit from adjuvant chemotherapy in completely resected patients: discovery of a holy grail?
The histologic hallmark of the majority of resected lung adenocarcinoma is their morphologic heterogeneity under the microscope (1-3). For many years pathologists have searched for clinically meaningful ways to classify resected lung adenocarcinoma but with little success (4). It is only recently that this heterogeneous “beast of many faces” has been tamed and linked to patient survival.
In 2011, an international multidisciplinary panel of lung cancer experts under the auspices of the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS) and European Respiratory Society (ERS), developed and recommended a novel classification system in which resected lung adenocarcinoma was to be classified according to the predominant histologic subtype, after identification and quantification of all histologic patterns present in the tumor in 5% increments and recognition of the predominant histologic pattern (5), a process termed comprehensive histologic subtyping (1). A flood of validation studies followed, most of which confirmed the prognostic impact of individual adenocarcinoma subtypes when predominant in a tumor (2,3,6-12). Furthermore many of these studies showed that grouping of adenocarcinoma subtypes with similar survival strengthened the prognostic impact of the classification (2,3,6). Hence, for the first time, it became possible to identify groups of patients with good prognosis tumors specifically those that are lepidic predominant, intermediate prognosis tumors including both acinar and papillary predominant tumors, and poor prognosis tumors including both micropapillary and solid predominant tumors. Importantly, recognition of individual adenocarcinoma patterns (13) and predominant histologic subtype (14,15) were demonstrated to be reasonably reproducible amongst groups of expert pulmonary pathologists, with improvement in kappa coefficients seen after training of pathologists with less lung cancer pathology experience in one study (14,16). In addition multiple studies reported correlations between predominant histologic subtype and various molecular abnormalities (8,17-20), although it is not currently recommended to select patients for molecular testing based on the predominant histologic subtype in their tumors (21). The 2011 IASLC/ATS/ERS has now been accepted as the basis for the classification of lung adenocarcinomas in the recently published fourth edition 2015 World Health Organisation (WHO) classification of lung tumours (22).
The search for patients with completely resected non-small cell lung cancer (NSCLC) who will benefit from adjuvant chemotherapy represents a holy grail in lung cancer treatment paradigms. This is because greater than 50% of patients with completely resected early stage NSCLC will develop recurrence after surgery (23) and most of those will die of their disease. The current staging system accurately predicts the risk of recurrence or death overtime for patients with a given stage (24), but does not provide any guide as to which patients will have relapse prevented by or delayed by adjuvant by chemotherapy.
The Lung Adjuvant Cisplatin Evaluation Biomarker (LACE-Bio) collaborative group was assembled in 2008 to perform validation studies or pooled analyses of biomarkers in a large cohort of patients participating in four adjuvant chemotherapy trials: the International Adjuvant Lung Cancer Trial (IALT), Adjuvant Navelbine International Trialist Association 01 (ANITA), JBR.10, and Cancer and Leukemia Group B (CALGB) 9633, now Alliance for Clinical Trials in Oncology studies (23). The main findings of the LACE-Bio meta-analysis were an 11% reduction in the risk of death at 5 years with the addition of adjuvant chemotherapy following complete resection of NSCLC and a significant stage interaction with benefit from adjuvant chemotherapy seen in patients with stages II and III NSCLC only. Unplanned post-hoc analyses identified a potential for a moderate but statistically significant benefit of chemotherapy in stage IB patients whose tumors were >4 cm in diameter (25).
In the 2008 LACE-Bio meta-analysis, tumors were histologically stratified into broad NSCLC subtypes including squamous cell carcinoma, adenocarcinoma and other, with no variation of chemotherapy effect seen with histologic subtype (23). Given that greater than 90% of adenocarcinomas fell into the mixed subtype category according to the 2004 WHO classification (4), further prognostically meaningful stratification was not possible at the time. However, with the radical changes to the classification landscape of lung adenocarcinoma, some lung cancer experts speculated as to the potential findings of classifying the adenocarcinoma cases in the LACE-Bio meta-analysis according to predominant histologic subtype, which in fact was one arena in which the 2011 IASLC/ATS/ERS classification had not been tested. Therefore its potential utility in choosing patients for adjuvant chemotherapy in the setting of a clinical trial was unknown.
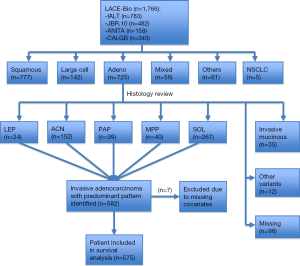
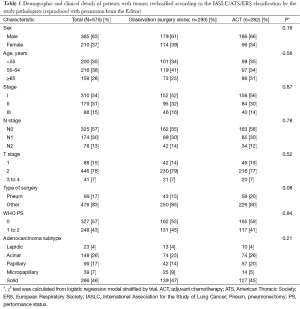
But this has all changed with very recent seminal work (26) led by internationally renowned pulmonary pathologists, Professor Ming-Sound Tsao from Princes Margaret Cancer Centre, Toronto, and Professor Elisabeth Brambilla from Centre Hospitalier Universitaire de Grenoble, Grenoble, both of whom were co-authors on the 2011 IASLC/ATS/ERS classification (4). These pathologists independently examined a cohort of 629 adenocarcinomas culled from the 725 original adenocarcinoma cases included in the LACE-Bio work. Figure 1 is a CONSORT (27) chart depicting patients with samples and molecular data available for the LACE-Bio meta-analysis. Of 629 adenocarcinoma cases with one representative H&E stained slide available for examination, 47 cases were excluded as variants and seven were excluded due to missing covariates. The remaining 575 cases were re-classified by the study pathologists using the new IASLC/ATS/ERS classification and included for survival analysis, resulting in 23 with lepidic predominant tumors, 148 with acinar predominant tumors, 99 with papillary predominant tumors, 39 with micropapillary predominant tumors, and 266 with solid predominant tumors (Table 1). Further clinical and demographic details of the patient groups are shown in Table 1, with 293 patients in the observation/surgery only arm and 282 patients in the surgery/adjuvant chemotherapy arm.
Full table
The first main finding from the reclassification of the 575 adenocarcinomas according to predominant histologic subtype relates to the correlation between survival and predominant subtype in the 293 patients in the observation arm. For this evaluation, the predominant subtypes were collapsed into three groups comprising a good prognosis group of lepidic predominant tumors, an intermediate prognosis group of acinar and papillary predominant tumors and a poor prognosis group of micropapillary and solid predominant tumors. On univariate analysis, there was a direction of effect towards a prognostic difference between the three subtype groups for overall survival (OS) and significant differences observed for disease-free survival (DFS), and for specific disease-free survival (SDFS), with solid and micropapillary predominant tumors experiencing worse outcomes. Similar results were obtained when all five predominant histologic subtypes were examined separately with no significant association for OS observed but significant associations demonstrated for DFS and SDFS. On multivariate survival analyses, no significant association was obtained for OS for acinar and papillary predominant tumors vs. lepidic predominant tumors, or for OS for micropapillary and solid predominant tumors vs. lepidic predominant tumors (Table 2). However marginally significant associations were observed for both DFS and SDFS with worse prognosis experienced for micropapillary and solid predominant tumors versus lepidic predominant tumors (Table 2). The authors note that the marginally significant differences seen for both DFS and SDFS were mostly due to the difference between acinar and papillary predominant tumors as the reference and micropapillary and solid predominant tumors. No heterogeneity of hazard ratios was seen across the trials.

Full table
The second main finding relates to the correlation between predominant histologic subtype and the effect of adjuvant chemotherapy in 552 patients, after exclusion of the 23 patients with lepidic predominant tumors. On univariate analysis, there was no significant benefit for adjuvant chemotherapy in patients with acinar and papillary predominant tumors for OS, DFS or SDFS. However, there was a non-significant direction of effect towards a benefit for adjuvant chemotherapy in patients with micropapillary and solid predominant tumors for OS and a significant benefit for DFS and SDFS. On multivariate analyses, there was a marginally significant benefit for adjuvant chemotherapy for OS for patients with micropapillary and solid predominant tumors but not for patients with acinar or papillary predominant tumors (Table 3); but this was dampened, as the treatment by histology interaction did not show significance. However, there was a significant benefit observed from adjuvant chemotherapy for patients with micropapillary and solid predominant tumors for DFS and SDFS but not for patients with acinar or papillary predominant tumors for DFS or SDFS (Table 3).

Full table
Therefore these data suggest that patients with solid and micropapillary predominant tumors experienced worse survival in comparison to patients with lepidic, acinar or papillary predominant tumors, and that it is this same group of patients—those with micropapillary and solid predominant tumors—who gained benefit with the addition of adjuvant chemotherapy. This is of enormous clinical relevance and supports previous recent studies (2,3,6), which have consistently shown that solid and micropapillary predominant tumors are the two predominant subtypes with the worst survival outcomes. In addition, solid predominant tumors are one of the more frequent subtypes in many published cohorts (3,6,8,11). Furthermore the supplementary data showed the treatment effect size for OS, DFS and SDFS was fairly consistent across all stages.
A major limitation of this work, apart from its retrospective nature and the small size of some of the pathologic groups, is that there was only one H&E stained slide from each case to review, which may not have been representative of the entire tumor. This limitation is one of the likely causes of trial heterogeneity seen in the meta-analysis. The IALT trial, in particular, shows no treatment effect or conflicting treatment effect compared to the other trials. It must be remembered that this trial recruited between the years of 1995 and 2000 and was designed as a “real world” trial to enhance recruitment. Centres had widely variable treatment policies on choice of chemotherapy agent (other than cisplatin) and criteria for adjuvant radiation. The only pathological requirement was documentation of NSCLC according to the 1981 WHO classification and most centres contributed fewer than 10 cases. Thus, we have to remain highly sceptical about the accuracy of histological subtyping based on just one slide from this large study.
It should also be noted that once cases have been divided by histological subtyping and then again by stage, the numbers being compared in this post-hoc study are relatively small, so a prospective study is still needed to determine if chemotherapy benefit by histology is independent of stage or perhaps even magnified by stage. It is plausible that minimal effect is seen in very early stage tumors due to infrequent relapse events, or in very locally advanced stage tumors due to generally more aggressive metastatic behaviour. It may be that it is patients with minimal nodal involvement tumors or high T-stage tumors in whom best selection for adjuvant chemotherapy by comprehensive histological subtyping occurs.
In conclusion, recent interrogation of the LACE-Bio work including patients from four randomized adjuvant chemotherapy trials by Professors Tsao and Brambilla and colleagues suggests that classification of lung adenocarcinoma according to the predominant histologic subtype as recommended by the 2011 IASLC/ATS/ERS classification and 2015 WHO classification of lung tumours may be of use in selecting patients with completely resected lung adenocarcinoma for adjuvant chemotherapy. Specifically, benefit for DFS and SDFS, but not for OS, was seen for patients with micropapillary and solid predominant tumors. This is of great clinical relevance as most studies investigating the correlation between survival and predominant histologic subtype have shown that micropapillary and solid predominant tumors have worse outcomes. Moreover solid predominant tumors are one of the more frequent histologic subtypes in many of these studies. The authors suggest that classification of lung adenocarcinomas according to predominant histologic subtype should be routinely used in adjuvant chemotherapy trials and that prospective validation studies are needed in the future.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by the Section Editor Jianfei Shen, MD (Department of thoracic Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al., editors. WHO Classification of Tumours 2004: Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press, 2004.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [PubMed]
- Mansuet-Lupo A, Bobbio A, Blons H, et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest 2014;146:633-43. [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [PubMed]
- Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J 2012;40:1221-7. [PubMed]
- Duhig EE, Dettrick A, Godbolt DB, et al. Mitosis trumps T stage and proposed international association for the study of lung cancer/american thoracic society/european respiratory society classification for prognostic value in resected stage 1 lung adenocarcinoma. J Thorac Oncol 2015;10:673-81. [PubMed]
- Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Arch 2012;461:185-93. [PubMed]
- Shim HS. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135:1329-34. [PubMed]
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53. [PubMed]
- Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461-8. [PubMed]
- Rekhtman N, Ang DC, Riely GJ, et al. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol 2013;26:1307-19. [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn 2013;15:415-53. [PubMed]
- Travis WD, Brambilla E, Burke A, et al., editors. WHO Classification of the Tumours of the Lung, Pleura, Thymus and Heart (ed 4). Lyon, France: IARC Press, 2015.
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Bonomi M, Pilotto S, Milella M, et al. Adjuvant chemotherapy for resected non-small-cell lung cancer: future perspectives for clinical research. J Exp Clin Cancer Res 2011;30:115. [PubMed]
- Tsao MS, Marguet S, Le Teuff G, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol 2015;33:3439-46. [PubMed]
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063-70. [PubMed]


