Molecular targeted therapy to improve radiotherapeutic outcomes for non-small cell lung carcinoma
Introduction
Radiotherapy (RT) plays an important role in the management of lung cancer. RT has an established role not just as an adjuvant therapy to surgery in the curative setting, but also in the definitive setting (as in the use of stereotactic RT for early stage lesions) and in the palliative role (involving treatment of metastatic lesions as well as in facilitating relief from compressive symptomatology) (1,2).
Since the beginning of the era of RT, there has been a quest to enhance outcomes—both by increasing the efficacy of RT, and by reducing radiation associated toxicities. The earlier years witnessed the use of altered fractionation to improve therapeutic ratio, later there had been experimentation with the use of chemotherapy (both sequential and concurrent) to enhance RT. Concurrent chemoradiotherapy with agents such as carboplatin, cisplatin, paclitaxel, docetaxel are known to enhance response rates, but also while including severe toxicities. The toxicities are sometimes severe to an extent so as to make it unusable in many patients of lung cancer since a large proportion of these patients entail co-morbidities such as diminished respiratory functions, cardiac issues, and age related issues (3).
In the recent years, there has been a significant breakthrough in the radiotherapeutic management of cancer. The use of targeted therapies in concurrent use with RT was seen as an effective approach, while being less toxic than the use of concurrent chemotherapy with RT. The success with the use of cetuximab in concurrent use with RT for head-neck squamous cell carcinoma ultimately led to the experimentation of a similar approach in other malignancies, including non-small cell lung carcinoma (NSCLC) (4). Almost simultaneously, there had been an interest in the use of anti-VEGF targeted therapies in concurrent use with RT (5,6). The discovery of oral tyrosine kinase inhibitors for certain types of NSCLC ushered in unprecedented convenience and efficacy (7,8).
It is hoped that evolution of targeted therapies for lung cancer can open up a new era in the radiotherapeutic management for lung cancer, with multiple experiments evaluating ways of integrating targeted therapy and RT for achieving synergy. While there is no dearth of theoretical targets, the lack of availability of clinically effective agents against these targets has been a source of frustration. This review discusses the current state of research in regards to the use of targeted therapies in concurrent use with RT for NSCLC.
A brief history of targeted therapies for lung cancer
Limitless replicative potential, growth self-sufficiency, anti-apoptotic potential, angiogenesis and the potential for invasion and metastasis are regarded as the ‘hallmarks of cancer’. The mentioned abilities are the result of dysregulation of signalling pathways either due to oncogene activation, or via a loss of tumor suppressive gene function. Oncogene activation could imply gene amplification, rearrangements, and point mutations. Loss of tumor suppressor gene function could be due to loss of heterozygosity or by epigenetic transcriptional silencing. Though both ‘oncogene activation’ and ‘loss of tumor suppressor gene function’ are known to be involved in the etiopathogenesis of lung cancer, there has been a greater understanding upon the mechanisms of oncogene activation and there exist opportunities at targeting the same (9-12).
The study of the phenomenon of oncogene activation led to the discovery of ‘oncogene addiction’ wherein a ‘driver oncogene’ is crucial for the tumor cells’ survival and proliferation. The commonly activated driver oncogenes in lung cancer include EGFR, KRAS, HER2, MYC, MET, EML4-ALK and BCL2. Since the targeting of a ‘driver oncogene’ would lead to specific killing of the ‘oncogene addicted’ tumor cells, these ‘driver oncogenes’ can in a way be regarded as the ‘Achilles heel’ of the tumor (13,14).
Clinically, EGFR mutations are the most common in lung cancer, and are of special interest due to the availability of multiple drugs to target EGFR. EGFR is a member of a family of transmembrane receptor kinases which also includes HER2, HER3 and HER4. EGFR and its associated receptor family are necessary for survival and are involved in maintenance of tissues including skin, heart, lungs and the central nervous system. Thus, it is not surprising that mutations of EGFR are oncogenic. The prevalence of EGFR mutations in lung cancer are difficult to estimate as it varies with ethnicity, sex and smoking status. Overall, EGFR mutations are expected in about 20–40% of Asian NSCLC patients. Mutations involving the kinase domain region (located from exon 18–21) of EGFR gene are ‘activating mutations’ since these mutations result in constitutive kinase activity of the receptor kinase, conferring ability of auto-activation (15,16).
Initial studies (such as BR.21 & INTEREST) evaluated EGFR tyrosine kinase inhibitors in NSCLC patients who had received prior treatment with chemotherapy, and without regards to either the patient’s histopathology or the EGFR mutation status. Despite this, there was an evidence of benefit with the use of gefitinib/erlotinib in comparison to placebo/chemotherapy (17,18).
The phase-III OPTIMAL trial was conducted to evaluate the PFS benefit with the use of erlotinib versus chemotherapy with gemcitabine-carboplatin. When used as first-line treatment in Chinese patients with EGFR mutated NSCLC. The median progression free survival (PFS) was better with erlotinib in comparison to chemotherapy (13.1 vs. 4.6 months; P<0.0001). These results were confirmed in the EURTAC study involving European patients (19,20).
While gefitinib and erlotinib represent oral TKIs which are effective against mutated EGFR, there exists an older class of targeted therapy agents, namely ‘monoclonal antibodies’. Monoclonal antibodies act on the extracellular aspect of the receptor, unlike the tyrosine kinase inhibitors which act on the intracellular domain. The anti-EGFR monoclonal antibody has already proven efficacy in patients of head-neck squamous cell cancers and colorectal adenocarcinomas (21-23). Their use in lung cancer has rather been an extrapolation based upon results in other sites. While cetuximab has been the most commonly used anti-EGFR monoclonal antibody in use, newer agents include panitumumab and nimotuzumab which are expected to provide similar efficacy at lesser toxicity as they have a diminished murine component in comparison to cetuximab (24-26).
Next to EGFR, the second mutation of particular importance happens to be the translocation mutation EML4-ALK, which is a lot less common in comparison to EGFR. Despite constituting just 3–6% of lung adenocarcinoma, it is of special interest because of the availability of an effective agent, namely crizotinib to target EML4-ALK mutation (27,28).
The VEGF pathway can be blocked by using monoclonal antibodies targeting VEGF, the use of VEGF receptor inhibitors (aflibercept), and by the use of small molecule tyrosine kinase inhibitors such as sunitinib and sorafenib to target the tyrosine kinase domain of VEGF receptor. The ECOG 4599 and the European AVAIL were two large phase III trials which helped gain approval for bevacizumab use in lung cancer, but strictly to be avoided in squamous cell carcinoma histology. Toxicities such as hemorrhage, esophageal toxicity could be severe. The results with aflibercept for platinum and erlotinib resistant lung cancer have been far from satisfactory in phase II trials. Small molecule tyrosine kinase inhibitors pazopanib, sunitinib, sorafenib and mosatenib are yet to be proven for safety and efficacy in phase III trials (29-32).
More targets such as KRAS, BRAF, MET, ILGF-1 and others are foci of on-going research, with no major data available for drawing impressions at this time (33-38).
Rationale for combining targeted therapies & RT
The combination of EGFR inhibitors with RT for NSCLC has strong theoretical rationale, as well as the backing of a body of evidence that can be interpolated from other sites such as head-neck & colorectal (39,40). RT induced tissue damage leads to increased EGFR expression which may be contributory to the dreaded phenomenon of accelerated tumor cell repopulation. Anti-EGFR monoclonal antibodies are especially effective in situations involving EGFR over-expression, thus rationalizing their use in concurrent use with RT. The use of anti-EGFR oral tyrosine kinase inhibitors is known to inhibit radioresistance by various mechanisms involving the cell growth pathways. It has been experimentally observed that anti-EGFR tyrosine kinase inhibitors are known to inhibit radioresistance by various mechanisms involving the cell growth pathways. It has also been observed that anti-EGFR tyrosine kinase inhibitors is known to inhibit radioresistance by various mechanisms involving the cell growth pathways including the reduction of percentage of tumor cells in the radioresistant ‘S-phase’ of the cell cycle, affect Rad51 expression, and reduce the radiation induced EGFR autophosphorylation (41). Also, the use of EGFR tyrosine kinase inhibitors in patients with EGFR activating mutations may lead to a rapid regression, hence reducing hypoxia and enhancing radiosensitivity (42-44).
The tumor vasculature is markedly disorganized in comparison to normal vasculature. The altered tumor vascular endothelium may lead to hypoxia, which not only causes increased radioresistance, but also promotes distant metastases. Also, RT is known to induce an increase in VEGF. Thus the use of anti-angiogenic therapy in concurrent use is rational, at least from a theoretical standpoint (45-47).
Existing experience on targeted therapies in use with RT
RT with anti-EGFR monoclonal antibodies
The first anti-EGFR monoclonal antibody to be used with RT is cetuximab. Cetuximab is a chimeric monoclonal antibody (partly murine, partly human), thus holding an occasional risk of allergic reaction. Newer agents include nimotuzumab and panitumumab. Nimotuzumab being a ‘humanized’ monoclonal antibody has modified protein sequences to increase similarity to human antibodies. Panitumumab is a fully human monoclonal antibody. While all of three agents act on the same target (the EGF receptor), the difference lies in the extent of expected toxicities. Further, cetuximab being an IgG1 may have the ability to activate complement pathway and cause antibody dependent cellular cytotoxicity, a feature which may theoretically be lacking in the IgG2 antibodies such as panitumumab. It is unknown at this time as to whether newer molecules (nimotuzumab & panitumumab) are equally effective as cetuximab, though newer molecules are likely to be less toxic (48-50).
The use of anti-EGFR monoclonal antibodies in concurrent use with RT has been summarized in Table 1. Though a pooled interpretation is difficult due to the varying complexity of study designs, the following inferences can be drawn at this time—that the use of anti-EGFR monoclonal antibodies in unresectable NSCLC is a good alternative to concurrent chemotherapy in patients unlikely to tolerate concurrent chemotherapy during RT; that the addition of anti-EGFR monoclonal antibodies when concurrent chemotherapy is already being used may not lead to additional benefit (as also observed in the scenario with head & neck squamous carcinoma); that radiation dose escalation may not translate to any benefit; and that anti-EGFR monoclonal antibodies may be effective as radiosensitizers in all NSCLC histologies, even if mutational status is not specifically known (51-59).

Full table
RT with EGFR tyrosine kinase inhibitors
While anti-EGFR monoclonal antibodies seem to be active in proportion to the level of EGFR expression, the activity of EGFR tyrosine-kinase inhibitors depend upon the presence of specific activating mutation of the EGFR. Gefitinib and erlotinib are the approved EGFR tyrosine kinase inhibitors in use. These orally administered drugs offer the advantage of convenience, too. The experience with the use of oral EGFR inhibitors with RT is summarized in Table 2. It can be remarked at this time that unlike anti-EGFR monoclonal antibodies which can be used in all of NSCLC regardless of the EGFR mutation status, the use of EGFR tyrosine kinase inhibitors must be restricted to adenocarcinoma histology harboring EGFR activating mutations. Also, while addition of gefitinib/erlotinib to RT may be helpful, there seems to be no benefit with the addition of gefitinib/erlotinib when concurrent chemotherapy is already being utilized. Currently, lack of large volume of data is a serious issue which hinders the drawing of confluences. Also, many of the existing data is from trials which did not provide impetus to patient selection based on histology and mutational status (60-68).

Full table
RT with anti-angiogenic agents
About a decade ago, the approval of bevacizumab as an anti-VEGF monoclonal antibody had led to the emergence of high hopes (69,70). However, it was soon realized that the use of bevacizumab had to be strictly avoided in patients with squamous cell carcinoma histology and in those with central thoracic lesions due to serious toxicity risks. Even when used for patients with adenocarcinoma histology, the use with concurrent chemotherapy and RT has led to unacceptable toxicities such as esophagitis and pneumonitis, while not offering significant enhancements in outcome. At present, the use of bevacizumab in concurrent use with RT cannot be recommended for routine clinical use. While newer anti-angiogenic agents such as endostatin, sunitinib and sorafenib are now available, it must be stressed upon that they are yet unproven for safety and efficacy in the scenario of concurrent use with RT (71-73).
RT with other targeted agents
While anti-EGFR therapies have been the mainstay of effective targeted therapies for NSCLC, there are new novel agents in consideration for trials. Bortezomib is a proteasome inhibitor, already approved for use in multiple myeloma. Though was found to have demonstrated radio-sensitizing properties in vitro, it was found un-safe for clinical use in a phase-I trial combining bortezomib with RT and chemotherapy (74,75). Sirolimus, a MTOR inhibitor has been tested in a phase I trial involving RT & concurrent cisplatin (76). Though safety has been evaluated, definitive results on response and survival is awaited. Finally, trials in early phases are evaluating celastrol (HSP90 inhibitor), vorinostat (HDAC inhibitor), selumetinib (MAPK inhibitor) and olaparib (PARP inhibitor) for concurrent use with RT (77-80). Though many novel agents (Table 3) have demonstrated radio-sensitizing properties in vitro, it needs to be seen if the results can be translated clinically.
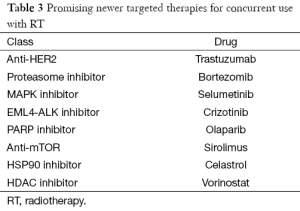
Full table
Conclusions
At present, it can be concluded that the use of anti-EGFR monoclonal antibodies for concurrent use with RT may be beneficial, and is an attractive option for NSCLC patients who are unable to tolerate concurrent chemotherapy for any reason. At the same time, it may be remarked that the addition of cetuximab when concurrent chemotherapy is already being provided with RT may not lead to any benefit. The use of EGFR tyrosine kinase inhibitors offers the convenience of the oral route of administration. However the use of EGFR tyrosine kinase inhibitors with RT is feasible only in adenocarcinoma patients with specific mutations. Anti-angiogenic therapy with RT may lead to more harm than benefit, and must be avoided at the present time. There are many newer agents against newer targets which are under investigation for concurrent use with RT. With painstaking and time consuming efforts, there will be hope for better results in the future.
Acknowledgements
The authors thank Dr Irappa Madabhavi, Medical Oncologist for his valuable and timely advice regarding the manuscript preparation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vinod SK. International patterns of radiotherapy practice for non-small cell lung cancer. Semin Radiat Oncol 2015;25:143-50. [PubMed]
- Tyldesley S, Boyd C, Schulze K, et al. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys 2001;49:973-85. [PubMed]
- De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol 2009;20:98-102. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [PubMed]
- Zhuang H, Zhao X, Zhao L, et al. Progress of clinical research on targeted therapy combined with thoracic radiotherapy for non-small-cell lung cancer. Drug Des Devel Ther 2014;8:667-75. [PubMed]
- Koh PK, Faivre-Finn C, Blackhall FH, et al. Targeted agents in non-small cell lung cancer (NSCLC): clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev 2012;38:626-40. [PubMed]
- Boolell V, Alamgeer M, Watkins DN, et al. The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers (Basel) 2015;7:1815-46. [PubMed]
- Zhou F, Zhou CC. Targeted therapies for patients with advanced NSCLC harboring wild-type EGFR: what's new and what's enough. Chin J Cancer 2015;34:310-9. [PubMed]
- Revannasiddaiah S, Thakur P, Bhardwaj B, et al. Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification. J Thorac Dis 2014;6:S502-25. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Weinstein IB. Cancer. Addiction to oncogenes-the Achilles heal of cancer. Science 2002;297:63-4. [PubMed]
- Perez R, Crombet T, de Leon J, et al. A view on EGFR targeted therapies from the oncogene-addiction perspective. Front Pharmacol 2013;4:53. [PubMed]
- Bronte G, Rizzo S, La Paglia L, et al. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev 2010;36:S21-9. [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [PubMed]
- Yano S, Kondo K, Yamaguchi M, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res 2003;23:3639-50. [PubMed]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol 2007;190:1-65. [PubMed]
- Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Johnson DE. Targeting proliferation and survival pathways in head and neck cancer for therapeutic benefit. Chin J Cancer 2012;31:319-26. [PubMed]
- Rivera F, García-Castaño A, Vega N, et al. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009;9:1421-8. [PubMed]
- Hubbard JM, Grothey A. Colorectal cancer in 2014: progress in defining first-line and maintenance therapies. Nat Rev Clin Oncol 2015;12:73-4. [PubMed]
- Reichert JM. Marketed therapeutic antibodies compendium. MAbs 2012;4:413-5. [PubMed]
- Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs 2009;1:41-8. [PubMed]
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569-79. [PubMed]
- Gridelli C, de Marinis F, Cappuzzo F, et al. Treatment of advanced non-small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer 2014;15:173-81. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [PubMed]
- Leighl NB, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 2010;5:1054-9. [PubMed]
- Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-smallcell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7. [PubMed]
- Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res 2012;18:1663-71. [PubMed]
- Takeuchi S, Wang W, Li Q, et al. Dual inhibition of Met kinase and angiogenesis to overcome HGF-induced EGFR-TKI resistance in EGFR mutant lung cancer. Am J Pathol 2012;181:1034-43. [PubMed]
- Bang YJ. Treatment of ALK-positive non-small cell lung cancer. Arch Pathol Lab Med 2012;136:1201-4. [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [PubMed]
- Landi L, Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther 2013;13:1219-28. [PubMed]
- Minuti G, D’Incecco A, Cappuzzo F. Targeted therapy for NSCLC with driver mutations. Expert Opin Biol Ther 2013;13:1401-12. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21-8. [PubMed]
- Weiss C, Arnold D, Dellas K, et al. Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: A pooled analysis of three prospective phase I-II trials. Int J Radiat Oncol Biol Phys 2010;78:472-8. [PubMed]
- Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005;65:3328-35. [PubMed]
- Tortora G, Gelardi T, Ciardiello F, et al. The rationale for the combination of selective EGFR inhibitors with cytotoxic drugs and radiotherapy. Int J Biol Markers 2007;22:S47-52. [PubMed]
- Kim JC, Ali MA, Nandi A, et al. Correlation of HER1/EGFR expression and degree of radiosensitizing effect of the HER1/EGFR-tyrosine kinase inhibitor erlotinib. Indian J Biochem Biophys 2005;42:358-65. [PubMed]
- Mehta VK. Radiotherapy and erlotinib combined: review of the preclinical and clinical evidence. Front Oncol 2012;2:31. [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [PubMed]
- Zhuang HQ, Yuan ZY. Process in the mechanisms of endostatin combined with radiotherapy. Cancer Lett 2009;282:9-13. [PubMed]
- Lind JS, Senan S, Smit EF. Pulmonary toxicity after bevacizumab and concurrent thoracic radiotherapy observed in a phase I study for inoperable stage III non-small-cell lung cancer. J Clin Oncol 2012;30:e104-8. [PubMed]
- Kim R. Cetuximab and panitumumab: are they interchangeable? Lancet Oncol 2009;10:1140-1. [PubMed]
- Patel D, Guo X, Ng S, et al. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum Antibodies 2010;19:89-99. [PubMed]
- Schneider-Merck T, Lammerts van Bueren JJ, Berger S, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010;184:512-20. [PubMed]
- Jatoi A, Schild SE, Foster N, et al. A phase II study of cetuximab and radiation in elderly and/or poor performance status patients with locally advanced non-small-cell lung cancer (N0422). Ann Oncol 2010;21:2040-4. [PubMed]
- Jensen AD, Münter MW, Bischoff HG, et al. Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer 2011;117:2986-94. [PubMed]
- Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8. [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [PubMed]
- Zaorsky NG, Sun Y, Wang Z, et al. Identification of a KRAS mutation in a patient with non-small cell lung cancer treated with chemoradiotherapy and panitumumab. Cancer Biol Ther 2013;14:883-7. [PubMed]
- Hallqvist A, Wagenius G, Rylander H, et al. Concurrent cetuximab and radiotherapy after docetaxel-cisplatin induction chemotherapy in stage III NSCLC: satellite--a phase II study from the Swedish Lung Cancer Study Group. Lung Cancer 2011;71:166-72. [PubMed]
- Chen Y, Moon J, Pandya KJ, et al. A Pilot Study (SWOG S0429) of Weekly Cetuximab and Chest Radiotherapy for Poor-Risk Stage III Non-Small Cell Lung Cancer. Front Oncol 2013;3:219. [PubMed]
- Choi HJ, Sohn JH, Lee CG, et al. A phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer 2011;71:55-9. [PubMed]
- Bebb G, Smith C, Rorke S, et al. Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer Chemother Pharmacol 2011;67:837-45. [PubMed]
- Ready N, Jänne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol 2010;5:1382-90. [PubMed]
- Choong NW, Mauer AM, Haraf DJ, et al. Phase I trial of erlotinib-based multimodality therapy for inoperable stage III non-small cell lung cancer. J Thorac Oncol 2008;3:1003-11. [PubMed]
- Niho S, Ohe Y, Ishikura S, et al. Induction chemotherapy followed by gefitinib and concurrent thoracic radiotherapy for unresectable locally advanced adenocarcinoma of the lung: a multicenter feasibility study (JCOG 0402). Ann Oncol 2012;23:2253-8. [PubMed]
- Okamoto I, Takahashi T, Okamoto H, et al. Single-agent gefitinib with concurrent radiotherapy for locally advanced non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Lung Cancer 2011;72:199-204. [PubMed]
- Center B, Petty WJ, Ayala D, et al. A phase I study of gefitinib with concurrent dose-escalated weekly docetaxel and conformal three-dimensional thoracic radiation followed by consolidative docetaxel and maintenance gefitinib for patients with stage III non-small cell lung cancer. J Thorac Oncol 2010;5:69-74. [PubMed]
- Rothschild S, Bucher SE, Bernier J, et al. Gefitinib in combination with irradiation with or without cisplatin in patients with inoperable stage III non-small cell lung cancer: a phase I trial. Int J Radiat Oncol Biol Phys 2011;80:126-32. [PubMed]
- Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimension conformal thoracic radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol 2008;3:250-7. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Wang J, Xia TY, Wang YJ, et al. Prospective study of epidermal growth factor receptor tyrosine kinase inhibitors concurrent with individualized radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e59-65. [PubMed]
- Nieder C, Wiedenmann N, Andratschke N, et al. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev 2006;32:348-64. [PubMed]
- Nieder C, Wiedenmann N, Andratschke NH, et al. Radiation therapy plus angiogenesis inhibition with bevacizumab: rationale and initial experience. Rev Recent Clin Trials 2007;2:163-8. [PubMed]
- Wachtel MS, Jumper CA, Halldorsson AO. Prognosis of metastatic carcinoma of the lung in the bevacizumab era: comparison between the major histologic types of lung cancer. J Surg Res 2012;174:20-3. [PubMed]
- Sandomenico C, Costanzo R, Carillio G, et al. Bevacizumab in non small cell lung cancer: development, current status and issues. Curr Med Chem 2012;19:961-71. [PubMed]
- Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008;26:60-5. [PubMed]
- Richardson PG, Mitsiades C, Hideshima T, et al. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med 2006;57:33-47. [PubMed]
- Edelman MJ, Burrows W, Krasna MJ, et al. Phase I trial of carboplatin/paclitaxel/bortezomib and concurrent radiotherapy followed by surgical resection in stage III non-small cell lung cancer. Lung Cancer 2010;68:84-8. [PubMed]
- Sarkaria JN, Schwingler P, Schild SE, et al. Phase I trial of sirolimus combined with radiation and cisplatin in nonsmall cell lung cancer. J Thorac Oncol 2007;2:751-7. [PubMed]
- Lee JH, Choi KJ, Seo WD, et al. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int J Mol Med 2011;27:441-6. [PubMed]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006;6:38-51. [PubMed]
- MEK Inhibitor and Thoracic Radiotherapy Trial (MEKRT). Available online: https://clinicaltrials.gov/ct2/show/NCT01146756
- Albert JM, Cao C, Kim KW, et al. Inhibition of poly(ADPribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 2007;13:3033-42. [PubMed]


