The relevance of molecular biomarkers in cervical cancer patients treated with radiotherapy
Introduction
Cervical cancer is a significant health problem for women around the world: it is the second most common female cancer (1), and the leading cause of cancer death in women in most developing countries (2). Human papillomavirus (HPV), the most common sexually transmitted infection worldwide, plays a major role in cervical cancer carcinogenesis, with HPV types 16 and 18 together accounting for about 70% of all cervical cancers (3). While early disease can be treated by radical hysterectomy, the standard treatment for advanced disease is surgery followed by radiotherapy (RT) with or without concurrent cisplatin chemotherapy (1) or radical concurrent chemoradiotherapy (CCRT) (4). Five-year survival rates are high for early stages, but decrease to 50-70% for stage IIB and only 30-50% and 5-15% for stage III and IV, respectively (5). Locoregional treatment failure is ascribed primarily to radioresistance: in stage IIB-III tumors, even high doses of 85 Gy result in 35-50% local failure (6). Moreover, radiation treatment failure is commonly associated with the development of metastases (7). Therefore, radioresistance is a clinically relevant problem in the management of cervical cancer.
The evaluation of biomarker status in cancers yields information about prognosis, treatment response, and vulnerability to targeted therapies. Historically, biomarker status in oncology was used in the differential diagnosis to differentiate cancer from normal tissue (8). However, rapid advances in genetic/genomic and histologic/proteomic technologies have led to a rapid expansion in biomarker functionality, ranging from estimating cancer risk [i.e., the breast and ovarian cancer mutation BRCA1 (9)] to predicting response to therapy [i.e., KRAS mutations in colorectal cancer (10); HER2 expression in breast cancer (11)].
The importance of radioresistance in cervical cancer treatment failure indicates that certain biomarkers may be useful for cervical cancer treatment individualization. Indeed, both translational and clinical research articles investigating this topic have increased dramatically in the past few years. However, the literature currently lacks a review of biomarker relevance in cervical cancer patients treated with RT. Therefore, we aimed to perform a review of the available literature to identify both clinically-validated intrinsic, non-secreted tumor cell protein biomarkers of radioresistance in cervical cancers, as well as putative biomarkers identified by laboratory experimentation in order to identify salient targets for further clinical investigation. This review aims to present validated tumor protein biomarkers that have pragmatic relevance to RT, and to discuss the biologic mechanisms of these biomarkers to better elucidate the cellular processes implicated in RT resistance.
Methods
From December 1983 to August 2015, PubMed was searched using the following queries:
- “Cervical carcinoma AND radioresistance” (85 results);
- “Cervical carcinoma AND biomarker radioresistance” (11 results);
- “Cervical carcinoma AND biomarker radiosensitivity” (35 results).
These queries yielded 131 results. Abstracts were screened for relevance. The full text of selected articles was read to assess for relevance and presentation of desired data. Studies were divided into clinical or basic science categories as defined below.
For the clinical studies reviewed, the following criteria were used for selection. Selected studies had to present evidence of a biomarker of radioresistance validated by immunohistochemistry for evaluation of expression levels in primary patient tumor samples. Radioresistance in these studies was defined as “poor or incomplete response to radiation therapy”, by “greater (than expected) rate of recurrence” after radiation therapy, or by poor overall survival (OS), metastasis-free survival (MFS), or disease-free survival (DFS) after radiation therapy compared to a reference radiosensitive group. Studies also needed to present patient survival or recurrence outcomes in biomarker vs. non-biomarker groups for a minimum of 3 years of follow-up. Studies also needed to include statistical significance of the association of the biomarker with a measure of poor prognosis.
The following criteria were used for the selection of basic science studies: studies could be carried out in either immortalized cervical cancer cell lines or primary cervical cancer cells. However, only those studies not presenting any patient outcome data were selected. Studies fulfilling these criteria were selected, and screened for quality.
Results
Studies presenting biomarkers validated in patient tumor data
Full-text screening and assessment for inclusion criteria yielded 19 publications that presented a biomarker of cervical cancer radioresistance validated using patient data (Table 1). Of these, 11 met the selection criteria. Eight studies presented survival data without univariate analysis (UVA) or hazard ratio (HR), and one included UVA and HR without survival outcome, and so were excluded.
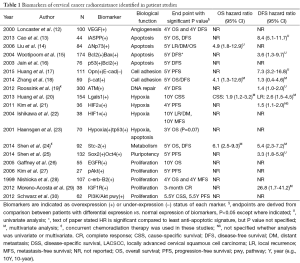
Full table
Cohort size ranged from 27 to 300. Seventeen of the 19 studies assessed radioresistance in patients treated with radiation alone, or presented data that distinguished patients treated with radiation alone separately from CCRT treated patients. All studies evaluated biomarker expression in patient samples via immunohistochemistry, except for Haensgen et al., which evaluated hypoxia via Eppendorf histograph rather than by a protein marker (23).
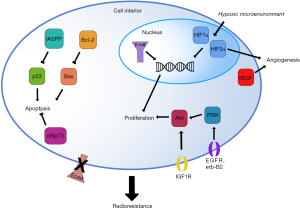
These 19 studies identified a total of 23 biomarkers (22 gene products and the 23th, hypoxia, a general characteristic of the tumor microenvironment) that can be separated into six biologic subgroups by the function of the protein: apoptosis, cell adhesion, DNA repair, hypoxia, metabolism, pluripotency, and proliferation. While the facile nature of these subgroups do not reflect the complex nature of some of these markers (for example, the complex roles of Akt and c-Erb-B2 in cell migration, metabolism, and apoptosis in addition to proliferation), these divisions are useful for a functional understanding of how the native protein product may be involved at the cellular level in the induction of radioresistance (Figure 1). Subgroups with the greatest representation and clinical relevance are discussed below.
Apoptosis evasion biomarkers
The ability to evade apoptosis is a hallmark of cancer cells of every origin (31). Of specific relevance to radiation therapy, radiation-induced apoptosis is a main mechanism of cancer cell killing in radiotherapeutic techniques (32,33). Expectedly, multiple biomarkers identified in this literature review are involved in the inhibition of apoptosis.
In response to various cellular stresses, including irradiation, pro-apoptotic Bax induces porosity of the outer mitochondrial membrane, and pro-apoptotic factors, notably cytochrome c and SMAC (an inhibitor of caspase inhibitors), invade the cytosol (34). These factors initiate, via activation of Casp9, proteolytic activation of a caspase cascade that cleave essential cellular proteins and thereby induce controlled cell death via the mitochondrial apoptotic pathway. p53, a master regulator of apoptosis, promotes apoptosis in response to DNA damage, UV exposure, stress, and other cytotoxic agents by several mechanisms including upregulation of Casp9 coactivators, transactivation of several death receptors including Fas, and shifting of the Bax/Bcl-2 balance to favor apoptosis (35).
Bcl-2
Bcl-2 controls cellular commitment to apoptosis by inhibiting the mitochondrial pathway. Bcl-2 interacts with targets such as Bax via its functional fold, defined by seven alpha-helices burying a central hydrophobic alpha-helix. Bcl-2 fold binding to Bax at Bax’s hydrophobic BH domains physically inhibits Bax signaling, blocking mitochondrial rupture and preventing caspase activation (34).
Several studies have identified Bcl-2 as an in vitro and in vivo marker of radiosensitivity in numerous malignancies. Recent work from the Haffty group has demonstrated a role for Bcl-2 in breast cancer radioresistance in vitro (36) and in vivo (37). Using the small-molecule Bcl-2 inhibitor ABT-737, a BH domain mimetic that binds Bcl-2 and prevents its binding and inactivation of Bax, in breast cancer cell lines MCF-7, ZR-75-1, and MDA-MB-231, Wu et al. (36) showed that Bcl-2 inhibition strongly sensitized cells to radiation, and that the mechanism of radiosensitization is via the down-regulation of pro-survival Mcl-1 and induction of the apoptotic Bak pathway. In a study of 116 patients receiving RT for early-stage breast cancer, Yang et al. demonstrated that Bcl-2 is an independent prognostic marker and a marker of radioresistance: Bcl-2 expression was associated with a significantly increased rate of local recurrence after RT (P=0.03) (37). Bcl-2 is also a clinically validated biomarker of radioresistance in small-cell lung cancer, multiple myeloma, several types of leukemia (38), laryngeal squamous cell carcinoma, and prostate cancer (39). Laboratory studies suggest a role in the radioresistance of additional malignancies: work from other groups further confirms that in breast cancer cells, treatment with ABT-737 reverses acquired radioresistance (40); and Bcl-2 overexpression in the hematopoietic system protects mice from normally lethal levels of radiation (41). This validation as a radioresistance biomarker in multiple cancer types coupled with the existence of ABT-737 and other inhibitors such as the orally bioavailable ABT-199 suggests that Bcl-2 status should be evaluated in cervical cancers and that Bcl-2 inhibition should be investigated for potential radiosensitization in these lesions (42).
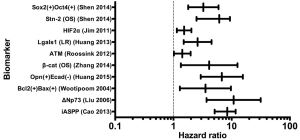
Two studies identified Bcl-2 as a biomarker of radioresistance in cervical cancers (Table 1) (15,16). In 174 cervical cancer patients treated with radiation, Wootipoom et al. demonstrated that tumors with the Bcl-2+/Bax+ signature had the worst DFS (HR =3.55; 95% CI, 1.29-9.72) (15) (Table 1 and Figure 2). Jain et al. (16) found that in 76 patients treated with RT, tumors with the p53(+)/Bcl-2(+) signature had poorer DFS (Table 1). Given that a major tumoricidal mechanism of radiation is the induction of apoptosis, the observation that tumors with increased Bcl-2 expression are resistant to radiation is not surprising. Although both of these studies demonstrated that Bcl-2 is not an independent marker of prognosis nor radioresistance, it remains a significant prognostic and radioresistance marker when evaluated in conjunction with Bax (15) or with tumor suppressor p53 (16). These observations likely reflect the fact that activation of the mitochondrial pathway is a multifactorial process dependent on the balance of diverse upstream inputs.
p73
p73 is a tumor suppressor homologue of p53, with a more complex role in oncogenesis. While some studies identify p73 as a tumor suppressor, others suggest that it functions as an oncogene (43); these contradictory observations are reconciled by the existence of several antagonistic splice variants of p73. Variant TAp73 drives apoptosis via activation of Bax in the mitochondrial apoptosis pathway (described above), whereas dominant-negative ΔNp73 inhibits both TAp73 and p53 to strongly repress Bax and inhibit apoptosis, analogous to Bcl-2 (44,45). Liu et al. demonstrated that upregulation of ΔNp73 in cervical tumors strongly predicts the likelihood of radioresistance (HR =10.8; 95% CI, 3.704-31.492), and predicts local recurrence (HR =4.857; 95% CI, 1.823-12.943) (14) (Table 1 and Figure 2). Moreover, ΔNp73 expression is associated with poorer 5-year OS (90% vs. 46% in ΔNp73 negative vs. n ΔNp73 positive cases, P<0.001). ΔNp73 may therefore impart radioresistance via inhibition of apoptosis through down-regulation of p53, TAp53, and Bax signaling.
iASPP
Compared to the well-characterized Bcl-2 and p73, the p53 inhibitor iASPP is less well-studied and understood. Genetic and biochemical studies in C. elegans and the human U2OS cell line demonstrate that iASPP inhibits p53-mediated apoptosis and that a physical interaction between iASPP and p53 exists, suggesting that iASPP exerts its oncogenic role by physically inhibiting p53 activity (46). Cao et al. showed that after resection and subsequent radiation, cervical cancer patients with high nuclear iASPP expression had shorter 5-year OS (66% vs. 100%, P=0.01) and 5-year DFS (53% vs. 90%, P=0.013) compared to patients with low nuclear iASPP (13) (Table 1 and Figure 2). Although a relationship between iASPP and radioresistance in other malignancies has not been studied, iASPP is associated with poor prognosis in melanoma (47), and drives proliferation of gastric (48), prostate (49), and tongue squamous carcinoma (50) cells in vitro, suggesting that iASPP should be further investigated in cervical and other cancers for prognostic and radiotherapeutic implications.
Hypoxia biomarkers
Inadequate oxygen supply, or hypoxia, is a characteristic of most tumors (51). Although malignant cells are defined by their ability to induce angiogenesis, the microvascular topology of a tumor is usually abnormal, with larger than normal intercapillary distances that are greater than the diffusion distance of oxygen (51). Hypoxia thus acts as a selective pressure that favors malignant pro-survival cell phenotypes such as migration, angiogenesis, and suppression of apoptosis, and also promotes genetic instability due to increased mitochondrial release of reactive oxygen species (ROS) (51,52). Furthermore, hypoxia directly opposes the efficacy of RT and yields radioresistance in tumor cells. Ionizing radiation is cytotoxic due to the induction of DNA double-strand breaks (DSBs); in hypoxic cells, the number of DSBs incurred upon irradiation is reduced, with anoxic cells displaying only 1/2 to 1/3 the DSBs of normoxic cells at a given dose, and the expression of DNA repair pathway components is also altered (53,54). Hypoxic conditions also induce expression of hypoxia-inducible factors (HIFs), transcription factors instrumental for survival in hypoxic conditions via inducing genes for angiogenesis, metabolism, invasion, proliferation, and metastasis, among other malignant phenotypes (55). Moreover, HIFs are essential to radioresistance in hypoxic lesions. Irradiation induces HIF expression, propagating the HIF survival phenotype and rendering further radioresistance (56); indeed, radiosensitization requires the presence of oxygen at the time of irradiation (53). Therefore, the innate hypoxic state in many tumors confers radioresistance, which is further aggravated by the upregulation of HIFs. Further confirming the well-demonstrated relationship between hypoxia and radioresistance is the identification of multiple molecular markers of hypoxia in the complement of radioresistance biomarkers in cervical cancer.
HIF1α and HIF2α
As discussed above, HIFs are up-regulated in response to both hypoxia and irradiation and induce multiple neoplastic attributes upon cancer cell. Ishikawa et al. identified HIF1α as a biomarker of poor prognosis after radiation therapy in cervical cancer patients; the 10-year recurrence-free survival (RFS) was only 22% in HIF1α positive tumors compared to 68.7% in HIF1α negative tumors (P=0.04) (22) (Table 1). Similar findings were reported by Kim et al. where HIF2α was significantly associated with a higher recurrence risk (HR =1.525; 95% CI, 1.144-2.033) (21) (Table 1).
Lgals1
While HIFs are well-characterized for their role in radioresistance, a relatively novel marker of hypoxia, Lgals1 (Galectin-1) is also implicated in cervical cancer radioresistance (57). Galectin-1 is a downstream transcriptional target of HIF1α, but also may participate dynamically in positive feedback upregulation of HIF1α via interactions with H-ras (57-59). In patients who received RT for cervical cancer, increased galectin-1 level was associated with a higher 10-year LR (HR =2.60; 95% CI, 1.50-4.52; P=0.0001) (Table 1 and Figure 2). This high galectin-1 expression patterns was similarly observed in relatively radioresistant tumors such as prostate cancer, melanoma, and glioma cells (57). In contrast, galectin-1 levels in Hodgkins lymphoma, a radiosensitive tumor, are decreased, but even within Hodgkins lymphoma subtypes, galectin-1 is lowest in patients with best prognosis (nodular lymphocyte type), and higher in patients with poorer prognosis markers (Reed Sternberg cells and anaplastic large-cell lymphomas) (57). These observations suggest that galectin-1 may drive radioresistance in resistant cancers, and that its synergistic relationship with HIF1α may perpetuate hypoxia-induced radioresistance.
Proliferation biomarkers
Uncontrolled proliferation is another hallmark of cancers of all types (31). Broadly, pro-proliferative cell surface receptors, cytosolic receptors, and intracellular signaling factors converge on or act directly as transcription factors to repress quiescence genes and/or stimulate proliferative genes. Indeed, many common oncogenes, such as the Ras superfamily, Myc, Abl, Cdks/cyclins, and numerous growth factor receptors such as the epidermal growth factor receptor (EGFR) and RET, are proliferative factors that allow cell division to proceed in an uncontrolled fashion. Accordingly, several of the biomarkers identified in this review are pro-proliferative factors up-regulated in radioresistant tumors. The strongest representatives of this class of biomarkers were members of the EGFR family and the Akt signaling pathway. Reinforcing the significance of these biomarkers being identified separately is that there is significant crosstalk between these pathways, affirming their role in radioresistance.
EGF and erbB receptors
EGFR and c-Erb-b2 are members of the large erbB family of receptor tyrosine kinases that drive proliferation through hormone/growth factor binding, activation, and a subsequent proliferative signal cascade. ErbB-family receptors exist as inactive monomers at the cell membrane; binding of their ligand, epidermal growth factor (EGF), initiates receptor dimerization, auto- and co-phosphorylation, and ultimately the activation of at least one downstream proliferative signaling pathway (60). Canonically, these pathways include the Ras-Raf-MAP kinase cascade and the PI3K/Akt pathways, both of which drive proliferation via upregulation of transcription and translation of pro-proliferative proteins (60). Furthermore, components of the ErbB family have been identified as oncogenes in numerous tumor types. HER2/neu (erb-B2/EGFR2) overexpression in breast cancer is perhaps the most well-known example, but EGF receptor dysregulation has also been identified in head and neck, ovarian, and colon carcinomas, among many others (61). The role of EGFR in cervical carcinoma is less well-defined. EGFR is expressed in the basal layer of normal cervical epithelium and in 50% to 70% of cervical squamous cell carcinomas, but studies of its association with progression, prognosis, and response to therapy are mixed, and its utility as a biomarker remains undefined for clinical use (62). However, an investigation of the predictive value of EGFR specifically for radioresistance in cervical cancer has not been performed until this review. Several studies demonstrate convincing clinical evidence that ErbB family proteins are associated with radioresistance in cervical cancer. Gaffney et al. demonstrated EGFR as a marker of radioresistance in primary cervical tumors (26). In 55 patients who received definitive RT for stage IB-IVA cervical carcinoma, patients with high EGFR staining had significantly poorer OS compared to patients with low-EGFR tumors (P=0.037) (Table 1). Multivariate analysis demonstrated a hazard ratio of 2.50 (95% CI, 1.24-5.05; P=0.011) predicting poorer OS in high-EGFR patients (Figure 2). Nishioka et al. characterized another EGFR isoform, c-erbB-2, for its role in radioresistance in cervical cancer (28). In 107 cervical carcinoma patients treated with RT, patients with c-erbB-2-positive tumors had significantly poorer OS in comparison to patients with low c-erbB-2 tumors (P=0.019), as well as significantly poorer MFS (70% vs. 33%; P<0.01) (Table 1). Identification of EGFR family members in two separate studies strongly supports the role of EGFR overexpression in cervical cancer radioresistance.
PI3K/Akt signaling
The PI3K/Akt signaling pathway is a major pro-proliferative, pro-survival, anti-apoptotic signaling pathway with upstream regulation by numerous growth and proliferation receptors. The activation of numerous growth factor receptors (including EGFR), insulin receptors, cytokine receptors, synaptic receptors, and immune cell receptors induces signaling through the PI3K/Akt pathway (63). This signaling is usually via direct activation of phosphoinositide-3 kinase (PI3K), which catalyzes the conversion of membrane phospholipids to PIP3, promoting phosphorylation and activation of Akt, also known as protein kinase B, through its kinase Pdk1. The kinase activity of Akt itself modulates the activity of the diverse targets mentioned above. Akt is a well-known oncogene; Akt itself is frequently mutated in ovarian (63,64), prostate, and breast cancers (65), and other components of the Akt pathway, including the tumor suppressor and opponent of PI3K, PTEN, are often mutated as well (66). The role of Akt signaling in cervical cancer has been largely unexplored. Several preliminary studies using small-molecule inhibitors of Akt have demonstrated that the inhibition of Akt signaling inhibits cervical cancer cell proliferation or promotes cancer cell death (67,68). Furthermore, Akt signaling is implicated in tumorigenesis in 3q-amplification, the most common chromosomal aberration in cervical cancers; this region of the long arm of chromosome 3 bears the catalytic subunit of Akt activator PI3K, and treatment of cervical cancer cell lines bearing this amplification with PI3K inhibitors decreases cell growth and promotes apoptosis (69).
Kim et al. explored the role of Akt activity in cervical cancer radioresistance (27). A total of 27 cervical carcinoma samples from patients who received RT were examined for Akt phosphorylation status (a direct indicator of Akt activation status). pAkt levels were significantly higher (P=0.004), and 5-year progression-free survival was significantly shorter (P=0.008), in radioresistant tumors compared to radiosensitive tumors (Table 1). These observations suggest that increased Akt activity can enhance the radioresistance of cervical tumors.
Work in the nascent field of gene signature profiling has also identified signaling activity in the PI3K/Akt pathway as a marker of cervical cancer radioresistance. Schwarz et al. used microarray-based gene expression quantification and gene set enrichment analysis to investigate the status of the PI3K/Akt pathway in radiosensitive vs. radioresistant cervical cancers (30). Components of the pathway, including Akt, PI3K, and nine others, were significantly overexpressed in radioresistant tumors (P=0.006) (Table 1). These observations were confirmed by immunohistochemistry for Akt expression levels in the corresponding tumors, and this measure of Akt pathway activity at the protein level independently confirmed that Akt is a marker of poorer progression-free survival and increased rate of recurrence. These observations further confirm that the Akt pathway is involved in cervical cancer radioresistance, making this pathway a salient target for further characterization. Furthermore, the complex relationship between EGFR signaling and the Akt pathway (Figure 1) supports a role for these pathways in radioresistance.
Cell adhesion biomarkers
Another defining characteristic of malignant cells is the ability to migrate from their niche and become invasive and metastatic, and a critical step in this process is the loss of cell-cell adhesion (31). A prime example in human carcinomas is the loss of epithelial adherens junction transmembrane protein E-cadherin (70). In normal epithelia, E-cadherin forms adherens junctions between adjacent epithelial cells, maintaining the integrity of the mucosal lining, preventing cell migration, and forbidding cells from exiting G0; predictably, the inhibition of E-cadherin promotes cellular migration, and E-cadherin loss promotes the epithelial-to-mesenchymal transition (EMT) (31). Accordingly, loss of E-cadherin has been demonstrated in carcinomas of the breast, gastrointestinal tract, pancreas, and numerous others (70), as well as in cervical squamous cell carcinoma (71-73). Another adherens junction protein, β-catenin, is an important component of cell adhesion. A unique membrane protein involved in both physical maintenance of cell adhesion as well as cytosolic signaling, β-catenin in its transmembrane form tethers E-cadherin’s intracellular domain to the actin cytoskeleton, promoting adhesion and quiescence in similar fashion to E-cadherin (74). However, translocation of β-catenin to the nucleus, where it acts directly as a transcription factor, promotes proliferation, and therefore β-catenin is an oncogene. Research on the role of β-catenin in cervical cancer is scarce, although work in transgenic mice has demonstrated that activated, nuclear β-catenin accelerates carcinogenesis in premalignant HPV-positive cervical lesions (75). In other cancer types, the EMT, to which E-cadherin loss contributes, has been implicated in radioresistance; examples include non-small cell lung cancer (17), prostate cancer (76), and others.
Two studies in human cervical patients implicate components of cell adhesion in radioresistance. In a study of 111 patients treated with radiation, Huang et al. demonstrated that concurrent loss of E-cadherin and overexpression of the epithelial cell transformation marker osteopontin is associated with significantly poorer progression-free survival at 5-year follow-up (Table 1 and Figure 2) (17). In another study, Zhang et al. identified an increase in nuclear β-catenin (i.e., upregulation of its oncogenic transcription factor function) as a biomarker of radioresistance (18). In 59 patients treated with RT, increased nuclear β-catenin was associated with poorer DFS (HR =1.3; 95% CI, 0.4-4.6), as well as poorer OS (HR =4.1; 95% CI, 1.3-12.6) (Table 1 and Figure 2). These studies implicate a role for aberrant cell adhesion in cervical cancer radioresistance.
Studies of putative biomarkers in laboratory experimentation
Investigation of potential biomarkers using cancer cell lines or primary cancer cells is a useful method of identifying potential biomarkers for further characterization in a clinical setting. Immortalized cancer cell lines are particularly useful because putative targets can be identified at less expense, and more rapidly. Subsequent clinical studies can then be targeted toward clinically relevant biomarkers. Laboratory studies are therefore essential for driving progress in the identification of clinically significant biomarkers. Clinicians interested in characterizing biomarkers of radioresistance in their cervical cancer patient populations should refer to the existing basic science literature to guide their selection of targets. To this end, we also present the biomarkers of radioresistance in cervical cancer identified in laboratory studies, highlighting those supported by the most salient and convincing data that clinicians may wish to characterize in a clinical study.
Screening for papers that presented high-quality laboratory evidence for a biomarker of radioresistance in cervical cancer not yet studied in patients yielded 16 results (Table 2). Five studies used post-radiation primary cervical cancer cells (from either post-radiation residual tumor, or from tumors otherwise demonstrated to be radioresistant). The remainder used either pre-treatment primary cervical cancer cells or one or more of the following immortalized: the cervical carcinoma lines C33A, CaSki, ME180, QG-U, SKG-I, SKG-II, or SiHa; and/or the cervical adenocarcinoma cell line HeLa. All studies that used pretreatment primary cervical cancer cells or immortalized cell lines used repeated irradiation to generate radioresistant subclones. These studies identified a total of 23 biomarkers of radioresistance in cervical cancer cells (Table 2). While, several of these studies identified radioresistance multigene signature, others demonstrated miRNA, epigenetic signature or protein product as key players in acquiring radioresistant features. Similarly to the clinical studies, these biomarkers can be categorized into eight groups by their general function: apoptosis, cell adhesion, cell cycle progression, cell motility, cytokine signaling, hypoxia, immunity, and proliferation or proliferation and inhibition of apoptosis.
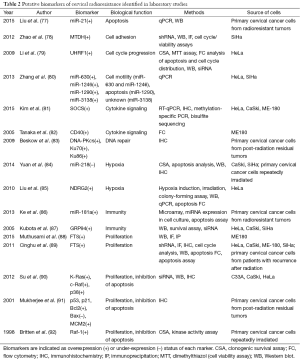
Full table
Most studies used similar techniques, directly evaluating biomarker expression status qualitatively (IHC) or quantitatively (qPCR, microarray, flow cytometry) after repeated irradiation. Several studies further validated biomarker status by using various knockdown techniques (shRNA or siRNA) to verify reduction of radioresistant phenotype after biomarker knockdown. Several studies also performed additional assays of radioresistance, including apoptosis assays, clonogenic survival assays, and various other cell cycle assays. All 16 studies provide convincing evidence for their biomarker’s role in radioresistance in cervical cancer, and we recommend that any of them be further investigated with prospective or retrospective clinical correlation. However, two studies of four biomarkers were exceptional in techniques used and the level of evidence presented for the biomarker’s role in radioresistance.
miR-181a
Ke et al. used both cell culture and mouse experiments to define the microRNA miR-181a as a biomarker of radioresistance in cervical cancer and to elucidate the mechanism of this radioresistance (86). miRNAs are endogenous small RNAs that reduce translation and decrease mRNA stability of their target genes by binding the 3’ untranslated region of their target mRNAs and inhibiting translation (93). Given that 30% of all genes may be regulated by miRNAs (94), they are likely relevant to many oncogenic and tumor suppressor processes. The miR-181 family of miRNAs includes miRNA-181b, miRNA-181c, and miRNA-181d in addition to miR-181a and it is involved in various endogenous functions, including T cell maturation (95) and vascular development (96). More importantly, miR-181 miRNAs are implicated in a number of cancers: miR-181a is a biomarker of multiple myeloma (97), and miR-181 family expression is associated with tamoxifen resistance in breast cancer (98). In this study, Ke et al. identified miR-181a as a potential radioresistance biomarker via microarray comparison of miRNA expression in primary cervical cancer samples obtained from radioresistant and radiosensitive tumors (86). The overexpression of miR-181a in SiHa and ME180 cell lines decreased their radiosensitivity, whereas treatment with miR-181a inhibitor increased cells radiosensitivity. To test the effect of miR-181a on actual tumor radioresistance in vivo, ME180 cell lines with the miR-181a overexpression vector were xenografted into nude mice, and mice were subjected to radiation treatment: mice with miR-181a tumors had significantly larger tumors after irradiation than mice with vector-control tumors. The group further defined the mechanism of this radioresistance, demonstrating that miR-181a inhibits apoptotic caspase activity in irradiated cells, promotes bypass of the G2/M block, and via genetic and biochemical approaches, showed that miR-181a inhibits apoptosis by silencing the protein kinase C isoform PRKCD; indeed, re-expression of PRKCD in the miR-181a overexpression background restored radiosensitivity to ME180 cells. The strong evidence for miR-181a as a biomarker of radioresistance in cervical cancer suggests that clinicians should consider pursuing investigations of its prognostic value and clinical implications of radioresistance.
EGFR, K-Ras, c-Raf, and p38
The Ras family of GTPases are the most commonly mutated oncogenes in human cancers (99); they drive proliferation via a mitogen-activated protein kinase (MAPK) cascade starting with their effectors, oncogenic Raf kinases (100). Another member of the MAPK family, p38, promotes cell migration (90). Upstream of the kinase cascade, EGFR activates Ras and itself is overexpressed in a plethora of human cancer types (101). Thus, EGFR signaling through the Ras/MAPK pathway is important in tumorigenesis, and correspondingly, these factors have been previously demonstrated as mediators of radioresistance in several mouse and rat cell lines (102,103), but their role specifically in cervical cancer radioresistance in human cases was not previously elucidated.
Su et al. investigated the role of the K-Ras pathway in radioresistance using experiments in cell culture and mice (90). Su et al. repeatedly irradiated CaSki, HeLa, and C33A cells to generate radioresistant subclones, and observed that migration was increased in the C33A and CaSki lines, measured by migration assays in culture as well as lung metastasis potential when injected into nude mice. This enhanced migration was dependent on K-ras, which was massively up-regulated in the radioresistant subclones and activated c-Raf in these clones. siRNA knockdown of either K-ras or c-raf separately obliterated the increased migration phenotype. siRNA or selective small-molecule inhibition of p38 similarly decreased radioresistant migration, suggesting that p38 is the downstream effector of Ras/Raf-mediated migration in radioresistant tumors. Evaluation of K-ras status in a small number of post-radiation distant metastases from cervical tumors paired with pre-radiation primary tumor showed that K-ras expression is much more strongly expressed in post-radiation distant metastases compared to primary tumor. These observations not only implicate Ras, Raf, and p38 in cervical cancer radioresistance, but provide a potential mechanism for the observation that many patients who fail radiation treatment exhibit distant metastases (7). Clinical validation of Ras, Raf, and p38 as biomarkers of cervical cancer radioresistance, to complement existing evidence for EGFR as a biomarker of radioresistance, and investigation of their association with treatment failure and poor prognosis would provide strong support for this theory, and should be pursued by clinicians.
Discussion
With the rapid expansion of the cervical cancer molecular literature, we examined the available clinical and laboratory studies to provide an updated review of radioresistance biomarkers in cervical cancer. Here we present a comprehensive analysis of the available high-quality literature investigating clinically-validated and putative biomarkers of radioresistance in cervical cancer. We identified 19 clinical studies describing 23 biomarkers of radioresistance validated by patient outcome data. We additionally identified 16 high-quality laboratory studies that identified 23 potential biomarkers of radioresistance that should be investigated in patient populations for outcome data. Biomarkers of radioresistance validated in patient studies included gene products functioning in apoptosis, cell adhesion, DNA repair, hypoxia, metabolism, pluripotency, and proliferation.
From our review, the most promising targets from clinical studies performed to date indicate that apoptosis proteins iASPP, ΔNp73, and Bcl-2, and hypoxia protein Lgals1 and HIF1α, induce radioresistance and are associated with poorer prognosis and merit further study. Select candidate biomarkers identified in laboratory studies should be further characterized in patient studies, particularly the miRNA miR-181a and proliferation/migration genes K-ras, c-Raf, and p38.
Additionally, further work is needed in specific areas to further our mechanistic understanding and our resultant treatment approach of biomarker-associated radioprotection.
Does HPV infection directly lend radiosensitivity to tumors, or is radiosensitivity conferred indirectly via modulation of other markers?
One accepted marker of cervical cancer radiation response is HPV status: low-risk HPV subtype positivity is associated with better OS, DFS, and local control (104-107). Interestingly, a similar association between response to radiation and HPV status has also been demonstrated in head and neck cancers, which can also be caused by HPV infection (108). HPV-positive head and neck squamous cell carcinomas (HNSCCs) have a favorable prognosis compared to HPV-negative HNSCCs when treated with radiation; the increased radiosensitivity in HPV-positive tumors is likely due to defective DNA DSB repair in HPV-positive tumors (109). However, the precise nature of a putative HPV-radiosensitivity relationship is not yet understood, whether in cervical malignancies or otherwise.
Several studies have suggested a relationship between HPV status and expression levels of potential biomarkers. Moreno-Acosta et al. identified insulin-like growth factor 1 receptor (IGF1R) as a biomarker of radioresistance in patients with HPV-16-positive cervical cancer (29). Interestingly, IGF1R has previously been identified as a marker of radioresistance in lung cancer (110), breast cancer (111), and osteosarcoma (112), as well as in cervical carcinoma of unknown HPV status (113). Moreno-Acosta’s observations suggest that interplay between HPV-16 and IGF1R may alter the radioresistance status of cervical tumors. Adding further complexity to the IGF1R narrative, Luo et al. showed that IGF1R levels are higher in cervical cancer tissues compared to paired normal tissues, and that increasing IGF1R expression in HPV-positive cervical cancer cell lines is inversely correlated with the expression of microRNA miR-497 (114). The loss of miR-497 has been implicated previously in the progression of HPV-positive HNSCC (115), so taken together; these observations suggest that HPV may drive radioresistance in cervical cancers by promoting IGF1R signaling, perhaps via suppression of miR-497 expression. Although these observations may seem to contradict the established relationship of HPV with favorable prognosis, the relationship is likely multifaceted; for instance, different isoforms of insulin-like growth factor, the ligand of IGF1R, have different downstream effects in different cancer types (116). Therefore, IGF1R is likely a significant biomarker of radiation response in HPV-positive cervical cancers, and its role in radioresistance or radiosensitivity should be further examined in future studies.
Seiki et al. showed that in HPV-16-positive cervical tumors, the protein phosphatase PP1γ is strongly mobilized from the nucleus, where it resides in HPV-negative tumors, to the cytosol, suggesting it is functionally inactive, and in vitro experiments in HPV-16-positive CaSki and SiHa cells demonstrated that HPV-16 (specifically, viral oncoproteins E6 and E7) is responsible for this relocalization and that E6/E7 mediate down-regulation of PP1γ expression (117). PP1 is of interest in that it is a major mediator of the DNA damage response and of appropriate mitotic exit, both pathways that are aberrant in cancers and particularly in radioresistant cancers (118). Furthermore, PP1 has been demonstrated to mediate radioresistance in non-small-cell lung cancer (119), making salient the model that HPV may modulate radioresistance through PP1γ. It is likely that a complex interplay between the HPV genome and endogenous factors conferring radiosensitivity or radioresistance exists; further exploration of the mechanism of HPV-induced radiosensitivity may reveal native radioresistance factors that are altered by HPV infection.
Conclusions
Advances in molecular profiling techniques have allowed for the identification and validation of biomarkers of numerous biological characteristics in tumor cells. Biomarkers of resistance or sensitivity to chemotherapeutic agents and RT are of interest for their potential role in designing customized therapeutic strategies targeting the vulnerabilities of individual tumors. We have discussed biomarkers of radioresistance in cervical carcinoma that have been validated in patient studies; further studies should better characterize the prognostic and predictive value of these markers to determine regimens for predicting response to RT. Potential biomarkers identified in laboratory studies should be further evaluated by clinicians in patient samples to identify additional markers of radioresistance in vivo.
Given that radiation is an integral component of the definitive treatment plan for cervical cancer (120), and that radiation treatment failure is common (121-123) and ascribed primarily to radioresistance (5), prediction of tumor response to radiation before the initiation of treatment would be invaluable for treatment planning. Traditional plans could be modified according to radioresistance status: patients with radioresistant tumors could be treated with protocols emphasizing surgery and chemotherapy, and radiosensitization strategies could be preferentially employed in radioresistant patients. To improve patient outcomes, clinicians should consider pretreatment evaluation of radioresistance biomarker status, and may also consider developing further studies investigation of the well-validated candidate biomarkers presented here.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Benedet JL, Bender H, Jones H 3rd, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet 2000;70:209-62. [PubMed]
- Peralta-Zaragoza O, Bermúdez-Morales VH, Pérez-Plasencia C, et al. Targeted treatments for cervical cancer: a review. Onco Targets Ther 2012;5:315-28. [PubMed]
- Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889-99. [PubMed]
- Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 2005.CD002225. [PubMed]
- Waggoner SE. Cervical cancer. Lancet 2003;361:2217-25. [PubMed]
- Perez CA, Grigsby PW, Chao KS, et al. Tumor size, irradiation dose, and long-term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys 1998;41:307-17. [PubMed]
- Huang EY, Wang CJ, Chen HC, et al. Multivariate analysis of para-aortic lymph node recurrence after definitive radiotherapy for stage IB-IVA squamous cell carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys 2008;72:834-42. [PubMed]
- Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol 2012;6:140-6. [PubMed]
- Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet 1995;56:265-71. [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [PubMed]
- Loncaster JA, Cooper RA, Logue JP, et al. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer 2000;83:620-5. [PubMed]
- Cao L, Huang Q, He J, et al. Elevated expression of iASPP correlates with poor prognosis and chemoresistance/radioresistance in FIGO Ib1-IIa squamous cell cervical cancer. Cell Tissue Res 2013;352:361-9. [PubMed]
- Liu SS, Chan KY, Cheung AN, et al. Expression of deltaNp73 and TAp73alpha independently associated with radiosensitivities and prognoses in cervical squamous cell carcinoma. Clin Cancer Res 2006;12:3922-7. [PubMed]
- Wootipoom V, Lekhyananda N, Phungrassami T, et al. Prognostic significance of Bax, Bcl-2, and p53 expressions in cervical squamous cell carcinoma treated by radiotherapy. Gynecol Oncol 2004;94:636-42. [PubMed]
- Jain D, Srinivasan R, Patel FD, et al. Evaluation of p53 and Bcl-2 expression as prognostic markers in invasive cervical carcinoma stage IIb/III patients treated by radiotherapy. Gynecol Oncol 2003;88:22-8. [PubMed]
- Huang X, Qian Y, Wu H, et al. Aberrant expression of osteopontin and E-cadherin indicates radiation resistance and poor prognosis for patients with cervical carcinoma. J Histochem Cytochem 2015;63:88-98. [PubMed]
- Zhang Y, Liu B, Zhao Q, et al. Nuclear localizaiton of β-catenin is associated with poor survival and chemo-/radioresistance in human cervical squamous cell cancer. Int J Clin Exp Pathol 2014;7:3908-17. [PubMed]
- Roossink F, Wieringa HW, Noordhuis MG, et al. The role of ATM and 53BP1 as predictive markers in cervical cancer. Int J Cancer 2012;131:2056-66. [PubMed]
- Huang EY, Chanchien CC, Lin H, et al. Galectin-1 is an independent prognostic factor for local recurrence and survival after definitive radiation therapy for patients with squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 2013;87:975-82. [PubMed]
- Kim MK, Kim TJ, Sung CO, et al. Clinical significance of HIF-2α immunostaining area in radioresistant cervical cancer. J Gynecol Oncol 2011;22:44-8. [PubMed]
- Ishikawa H, Sakurai H, Hasegawa M, et al. Expression of hypoxic-inducible factor 1alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2004;60:513-21. [PubMed]
- Haensgen G, Krause U, Becker A, et al. Tumor hypoxia, p53, and prognosis in cervical cancers. Int J Radiat Oncol Biol Phys 2001;50:865-72. [PubMed]
- Shen XJ, Gu K, Shi JP, et al. Increased expression of stanniocalcin 2 is associated with tumor progression after radiotherapy in patients with cervical carcinoma. Int J Clin Exp Pathol 2014;7:8770-6. [PubMed]
- Shen L, Huang X, Xie X, et al. High Expression of SOX2 and OCT4 Indicates Radiation Resistance and an Independent Negative Prognosis in Cervical Squamous Cell Carcinoma. J Histochem Cytochem 2014;62:499-509. [PubMed]
- Gaffney DK, Haslam D, Tsodikov A, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys 2003;56:922-8. [PubMed]
- Kim TJ, Lee JW, Song SY, et al. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer 2006;94:1678-82. [PubMed]
- Nishioka T, West CM, Gupta N, et al. Prognostic significance of c-erbB-2 protein expression in carcinoma of the cervix treated with radiotherapy. J Cancer Res Clin Oncol 1999;125:96-100. [PubMed]
- Moreno-Acosta P, Gamboa O, Sanchez de Gomez M, et al. IGF1R gene expression as a predictive marker of response to ionizing radiation for patients with locally advanced HPV16-positive cervical cancer. Anticancer Res 2012;32:4319-25. [PubMed]
- Schwarz JK, Payton JE, Rashmi R, et al. Pathway-specific analysis of gene expression data identifies the PI3K/Akt pathway as a novel therapeutic target in cervical cancer. Clin Cancer Res 2012;18:1464-71. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys 1995;33:781-96. [PubMed]
- Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res 2000;301:133-42. [PubMed]
- Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 2014;15:49-63. [PubMed]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene 2003;22:9030-40. [PubMed]
- Wu H, Schiff DS, Lin Y, et al. Ionizing radiation sensitizes breast cancer cells to Bcl-2 inhibitor, ABT-737, through regulating Mcl-1. Radiat Res 2014;182:618-25. [PubMed]
- Yang Q, Moran MS, Haffty BG. Bcl-2 expression predicts local relapse for early-stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat 2009;115:343-8. [PubMed]
- Juin P, Geneste O, Gautier F, et al. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer 2013;13:455-65. [PubMed]
- An J, Chervin AS, Nie A, et al. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene 2007;26:652-61. [PubMed]
- Li JY, Li YY, Jin W, et al. ABT-737 reverses the acquired radioresistance of breast cancer cells by targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res 2012;31:102. [PubMed]
- Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood 1998;91:2272-82. [PubMed]
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013;19:202-8. [PubMed]
- Stiewe T, Pützer BM. Role of p73 in malignancy: tumor suppressor or oncogene? Cell Death Differ 2002;9:237-45. [PubMed]
- Ramadan S, Terrinoni A, Catani MV, et al. p73 induces apoptosis by different mechanisms. Biochem Biophys Res Commun 2005;331:713-7. [PubMed]
- Grob TJ, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ 2001;8:1213-23. [PubMed]
- Bergamaschi D, Samuels Y, O'Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 2003;33:162-7. [PubMed]
- Pandolfi S, Montagnani V, Lapucci A, et al. HEDGEHOG/GLI-E2F1 axis modulates iASPP expression and function and regulates melanoma cell growth. Cell Death Differ 2015. [Epub ahead of print]. [PubMed]
- Wang LL, Xu Z, Peng Y, et al. Downregulation of inhibitor of apoptosis-stimulating protein of p53 inhibits proliferation and promotes apoptosis of gastric cancer cells. Mol Med Rep 2015;12:1653-8. [PubMed]
- Morris EV, Cerundolo L, Lu M, et al. Nuclear iASPP may facilitate prostate cancer progression. Cell Death Dis 2014;5:e1492. [PubMed]
- Chen Y, Yan W, He S, et al. In vitro effect of iASPP on cell growth of oral tongue squamous cell carcinoma. Chin J Cancer Res 2014;26:382-90. [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [PubMed]
- Guzy RD, Hoyos B, Robin E, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005;1:401-8. [PubMed]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008;8:180-92. [PubMed]
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437-47. [PubMed]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010;29:625-34. [PubMed]
- Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004;5:429-41. [PubMed]
- Huang EY, Chen YF, Chen YM, et al. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis 2012;3:e251. [PubMed]
- Elad-Sfadia G, Haklai R, Ballan E, et al. Galectin-1 augments Ras activation and diverts Ras signals to Raf-1 at the expense of phosphoinositide 3-kinase. J Biol Chem 2002;277:37169-75. [PubMed]
- Paz A, Haklai R, Elad-Sfadia G, et al. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001;20:7486-93. [PubMed]
- Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389-400. [PubMed]
- Yewale C, Baradia D, Vhora I, et al. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials 2013;34:8690-707. [PubMed]
- Soonthornthum T, Arias-Pulido H, Joste N, et al. Epidermal growth factor receptor as a biomarker for cervical cancer. Ann Oncol 2011;22:2166-78. [PubMed]
- Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 2012;4:a011189. [PubMed]
- Yuan ZQ, Sun M, Feldman RI, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene 2000;19:2324-30. [PubMed]
- Sun M, Wang G, Paciga JE, et al. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol 2001;159:431-7. [PubMed]
- Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455-64. [PubMed]
- Rashmi R, DeSelm C, Helms C, et al. AKT inhibitors promote cell death in cervical cancer through disruption of mTOR signaling and glucose uptake. PLoS One 2014;9:e92948. [PubMed]
- Wu J, Chen C, Zhao KN. Phosphatidylinositol 3-kinase signaling as a therapeutic target for cervical cancer. Curr Cancer Drug Targets 2013;13:143-56. [PubMed]
- Ma YY, Wei SJ, Lin YC, et al. PIK3CA as an oncogene in cervical cancer. Oncogene 2000;19:2739-44. [PubMed]
- Berx G, van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 2009;1:a003129. [PubMed]
- Fadare O, Reddy H, Wang J, et al. E-Cadherin and beta-Catenin expression in early stage cervical carcinoma: a tissue microarray study of 147 cases. World J Surg Oncol 2005;3:38. [PubMed]
- Chen CL, Liu SS, Ip SM, et al. E-cadherin expression is silenced by DNA methylation in cervical cancer cell lines and tumours. Eur J Cancer 2003;39:517-23. [PubMed]
- Vessey CJ, Wilding J, Folarin N, et al. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J Pathol 1995;176:151-9. [PubMed]
- Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J 2012;31:2714-36. [PubMed]
- Bulut G, Fallen S, Beauchamp EM, et al. Beta-catenin accelerates human papilloma virus type-16 mediated cervical carcinogenesis in transgenic mice. PLoS One 2011;6:e27243. [PubMed]
- Chang L, Graham PH, Hao J, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis 2013;4:e875. [PubMed]
- Liu S, Song L, Zhang L, et al. miR-21 modulates resistance of HR-HPV positive cervical cancer cells to radiation through targeting LATS1. Biochem Biophys Res Commun 2015;459:679-85. [PubMed]
- Zhao Y, Moran MS, Yang Q, et al. Metadherin regulates radioresistance in cervical cancer cells. Oncol Rep 2012;27:1520-6. [PubMed]
- Li XL, Meng QH, Fan SJ. Adenovirus-mediated expression of UHRF1 reduces the radiosensitivity of cervical cancer HeLa cells to gamma-irradiation. Acta Pharmacol Sin 2009;30:458-66. [PubMed]
- Zhang B, Chen J, Ren Z, et al. A specific miRNA signature promotes radioresistance of human cervical cancer cells. Cancer Cell Int 2013;13:118. [PubMed]
- Kim MH, Kim MS, Kim W, et al. Suppressor of cytokine signaling (SOCS) genes are silenced by DNA hypermethylation and histone deacetylation and regulate response to radiotherapy in cervical cancer cells. PLoS One 2015;10:e0123133. [PubMed]
- Tanaka T, Bai T, Yukawa K, et al. Reduced radiosensitivity and increased CD40 expression in cyclophosphamide-resistant subclones established from human cervical squamous cell carcinoma cells. Oncol Rep 2005;14:941-8. [PubMed]
- Beskow C, Skikuniene J, Holgersson A, et al. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer 2009;101:816-21. [PubMed]
- Yuan W, Xiaoyun H, Haifeng Q, et al. MicroRNA-218 enhances the radiosensitivity of human cervical cancer via promoting radiation induced apoptosis. Int J Med Sci 2014;11:691-6. [PubMed]
- Liu J, Zhang J, Wang X, et al. HIF-1 and NDRG2 contribute to hypoxia-induced radioresistance of cervical cancer Hela cells. Exp Cell Res 2010;316:1985-93. [PubMed]
- Ke G, Liang L, Yang JM, et al. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene 2013;32:3019-27. [PubMed]
- Kubota H, Suzuki T, Lu J, et al. Increased expression of GRP94 protein is associated with decreased sensitivity to X-rays in cervical cancer cell lines. Int J Radiat Biol 2005;81:701-9. [PubMed]
- Muthusami S, Prabakaran DS, Yu JR, et al. FTS is responsible for radiation-induced nuclear phosphorylation of EGFR and repair of DNA damage in cervical cancer cells. J Cancer Res Clin Oncol 2015;141:203-10. [PubMed]
- Cinghu S, Anandharaj A, Lee HC, et al. FTS (fused toes homolog) a novel oncoprotein involved in uterine cervical carcinogenesis and a potential diagnostic marker for cervical cancer. J Cell Physiol 2011;226:1564-72. [PubMed]
- Su WH, Chuang PC, Huang EY, et al. Radiation-induced increase in cell migration and metastatic potential of cervical cancer cells operates via the K-Ras pathway. Am J Pathol 2012;180:862-71. [PubMed]
- Mukherjee G, Freeman A, Moore R, et al. Biologic factors and response to radiotherapy in carcinoma of the cervix. Int J Gynecol Cancer 2001;11:187-93. [PubMed]
- Britten RA, Perdue S, Opoku J, et al. Paclitaxel is preferentially cytotoxic to human cervical tumor cells with low Raf-1 kinase activity: implications for paclitaxel-based chemoradiation regimens. Radiother Oncol 1998;48:329-34. [PubMed]
- Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol 2007;210:279-89. [PubMed]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15-20. [PubMed]
- Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 2007;129:147-61. [PubMed]
- Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 2010;116:2395-401. [PubMed]
- Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A 2008;105:12885-90. [PubMed]
- Lowery AJ, Miller N, Devaney A, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res 2009;11:R27. [PubMed]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11-22. [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84.
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007;26:3291-310. [PubMed]
- Bernhard EJ, Kao G, Cox AD, et al. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras-transformed rat embryo fibroblasts. Cancer Res 1996;56:1727-30. [PubMed]
- Sklar MD. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science 1988;239:645-7. [PubMed]
- Lindel K, Burri P, Studer HU, et al. Human papillomavirus status in advanced cervical cancer: predictive and prognostic significance for curative radiation treatment. Int J Gynecol Cancer 2005;15:278-84. [PubMed]
- Harima Y, Sawada S, Nagata K, et al. Human papilloma virus (HPV) DNA associated with prognosis of cervical cancer after radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1345-51. [PubMed]
- Vosmik M, Laco J, Sirak I, et al. Prognostic significance of human papillomavirus (HPV) status and expression of selected markers (HER2/neu, EGFR, VEGF, CD34, p63, p53 and Ki67/MIB-1) on outcome after (chemo-) radiotherapy in patients with squamous cell carcinoma of uterine cervix. Pathol Oncol Res 2014;20:131-7. [PubMed]
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16:1-17. [PubMed]
- Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol 2008;5:24-31. [PubMed]
- Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol 2013;107:242-6. [PubMed]
- Zhang H, Zhang C, Wu D. Activation of insulin-like growth factor 1 receptor regulates the radiation-induced lung cancer cell apoptosis. Immunobiology 2015;220:1136-40. [PubMed]
- Li P, Veldwijk MR, Zhang Q, et al. Co-inhibition of epidermal growth factor receptor and insulin-like growth factor receptor 1 enhances radiosensitivity in human breast cancer cells. BMC Cancer 2013;13:297. [PubMed]
- Wang YH, Wang ZX, Qiu Y, et al. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits growth, reduces invasion, and enhances radiosensitivity in human osteosarcoma cells. Mol Cell Biochem 2009;327:257-66. [PubMed]
- Lloret M, Lara PC, Bordón E, et al. IGF-1R expression in localized cervical carcinoma patients treated by radiochemotherapy. Gynecol Oncol 2007;106:8-11. [PubMed]
- Luo M, Shen D, Zhou X, et al. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery 2013;153:836-47. [PubMed]
- Lajer CB, Garnæs E, Friis-Hansen L, et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer 2012;106:1526-34. [PubMed]
- Christopoulos PF, Philippou A, Koutsilieris M. Pattern of IGF-1 variants' expression in human cancer cell lines using a novel q-RT-PCR approach. Anticancer Res 2015;35:107-15. [PubMed]
- Seiki T, Nagasaka K, Kranjec C, et al. HPV-16 impairs the subcellular distribution and levels of expression of protein phosphatase 1γ in cervical malignancy. BMC Cancer 2015;15:230. [PubMed]
- Wurzenberger C, Gerlich DW. Phosphatases: providing safe passage through mitotic exit. Nat Rev Mol Cell Biol 2011;12:469-82. [PubMed]
- Kim W, Youn H, Kang C, et al. Inflammation-induced radioresistance is mediated by ROS-dependent inactivation of protein phosphatase 1 in non-small cell lung cancer cells. Apoptosis 2015;20:1242-52. [PubMed]
- Viswanathan AN, Thomadsen B; American Brachytherapy Society Cervical Cancer Recommendations Committee; et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy 2012;11:33-46. [PubMed]
- Rajasooriyar C, Van Dyk S, Bernshaw D, et al. Patterns of failure and treatment-related toxicity in advanced cervical cancer patients treated using extended field radiotherapy with curative intent. Int J Radiat Oncol Biol Phys 2011;80:422-8. [PubMed]
- Ito H, Kutuki S, Nishiguchi I, et al. Radiotherapy for cervical cancer with high-dose rate brachytherapy correlation between tumor size, dose and failure. Radiother Oncol 1994;31:240-7. [PubMed]
- Jensen LG, Hasselle MD, Rose BS, et al. Outcomes for patients with cervical cancer treated with extended-field intensity-modulated radiation therapy and concurrent cisplatin. Int J Gynecol Cancer 2013;23:119-25. [PubMed]


