Effectiveness of anisodamine for the treatment of critically ill patients with septic shock (ACIdoSIS study): study protocol for randomized controlled trial
Introduction
Septic shock is an important contributor of mortality in the intensive care unit (ICU). The crude mortality is reported to be from 30% to 65% (1-5). Although there are significant advances in the management of septic shock in recent decades, the mortality rate was only marginally reduced. For example, the CUB-Réa Network study reported that the mortality rate of septic shock declined from 62.1% in 1993 to 55.9% in 2000 (6). The well-known Surviving Sepsis Campaign has also made every effort to reduce mortality rate of severe sepsis and septic shock. The organization recommended bundled strategies including early goal directed therapy (EGDT) for the management of septic shock (7,8). Although EGDT was once the mainstay therapy of septic shock, its efficacy has been questioned by recent several large randomized controlled trials (9,10). Therefore, the treatment of septic shock is still a global challenge and there is no well-established intervention that can reduce its mortality.
Anisodamine is an active agent isolated from a Chinese herb medicine. Both experimental and clinical studies have shown some potential beneficial effects of anisodamine in improving outcomes of shock (11-13). It was reported that anisodamine could reduce the mortality rate of fulminant epidemic meningitis from 66.9% to 12.4% (14). The efficacy of anisodamine might be mediated via the inhibition of thromboxane synthesis, granulocyte and platelet aggregation (15). Although anisodamine has been widely used in the treatment of septic shock in mainland China, there is no solid evidence from well-designed clinical trials to support its efficacy. The aim of the study is to investigate the effectiveness of anisodamine in the treatment of critically ill patients with septic shock.
Methods
Study design and setting
The study was a prospective randomized controlled trial that will recruit a maximum of 346 patients over a 2-3 years period. Patients with septic shock will be enrolled at participating hospitals in mainland China. Investigators in each participating center will screen patients with septic shock for potential eligibility. The study was reviewed and approved by the institutional review board of Jinhua Municipal Central Hospital (approval No. 2015-13) and the ethics committee of each participating center. Informed consent will be obtained from participants or their next-of-kin. The study was registered in the website ClinicalTrials.gov (registration No.: NCT02442440).
Participants
Inclusion criteria included patients with sepsis plus use of vasopressors. Systemic inflammatory response syndrome (SIRS) is defined as meeting at least one of the following 3 criteria for a systemic inflammatory response. One of the SIRS criteria must be either the WBC criteria (I) or the body temperature criteria (II):
- White blood cell count >12,000 or <4,000 or >10% band forms;
- Body temperature >38 °C (any route) or <36 °C (accepting core temperatures only; indwelling catheter, esophageal, rectal);
- Heart rate (>90 beats/min) or receiving medications that slow heart rate or paced rhythm;
- Tachypnea (>20 breaths per minute), or, an arterial partial pressure of carbon dioxide less than 4.3 kPa (32 mmHg).
Suspected or documented infection included the following sites: thorax, urinary tract, abdomen, skin, sinuses, central venous catheters, and bacterial meningitis.
Septic shock was defined as sustained arterial hypotension with systolic blood pressure (SBP) <90 mmHg, mean arterial pressure (MAP) <70 mmHg, or an SBP decrease >40 mmHg, despite adequate fluid resuscitation. To ease clinical screening process, we defined septic shock as the requirement of vasopressors despite adequate fluid resuscitation. Vasopressors include norepinephrine, epinephrine, phenylephrine and dopamine >5 mcg/kg/min.
Patients with following conditions will be excluded:
- Age <15 years old;
- Moribund (expected to die within 24 h);
- Stay in ICU for more than 24 h at enrollment;
- Contraindications to anisodamine: elevated intracranial pressure, acute phase of intracranial hemorrhage, glaucoma, untreated bowel obstruction (surgically treated obstruction is not contraindicated), enlargement of prostate without urinary catheterization.
Randomization
Blocked randomization was performed where anisodamine and control treatments were allocated at random in a ratio of 1:1 in blocks of sizes 2, 4, 6, 8, and 10 to 346 subjects. Block sizes are allocated unequally in the ratio 1:4:6:4:1 (Pascal’s triangle).
Investigators at each participating center will screen all potentially eligible patients. If there are eligible patients they will inform randomization center via the software Wechat (Tencent, China). The center will allocate a number to that patient within 6 h and indicate which group the patient will be allocated to. The investigators know the allocation and this study is an open label trial.
Treatment
A bolus of 10 mg anisodamine was given intravenously as the loading dose, followed by micro-pump at the rate of 0.1 to 0.5 mg/kg/h. The maintenance dose will be titrated at the discretion of the treating physician according the microcirculation status, as well as the side effects. For example, infusion rate can be increased if the serum lactate continues to elevate. Conversely, if the use of anisodamine results in significant drop in blood pressure, the infusion rate can be reduced.
Anisodamine will be discontinued on the recovery of shock (vasopressor discontinuation and normalized serum lactate), significant adverse events, and death.
The control group received usual care without anisodamine.
Study endpoint
The primary study end point is the hospital mortality, defined as death status at hospital discharge.
Secondary study endpoints include ICU mortality, length of stay in ICU and hospital, organ failure free days. Organ failure will be assessed by using sequential organ failure assessment (SOFA) score. SOFA scores will be calculated daily for the first 7 days.
Adverse events
Adverse events including new onset psychosis, urinary retention, significant hypotension and tachycardia will be reported.
Data collection
Research coordinators will collect data and record it on paper data forms. The case report form (CRF) is written in Chinese to facilitate communication among investigators. Data will be checked and obvious outliers or impossible entries will be recorded and discussed with site research coordinators. The site research coordinator will check the value and correct it if necessary.
Group sequential analysis
Sequential trial analysis will be performed. We planned to perform 6 interim analyses at the accrual sample size of 59, 118, 177, 236, 295 and 354 (Table 1). The trial may be stopped at early for efficacy or futility at respect adjusted significance levels. Asymmetric two-sided group sequential design will be performed with binding futility bound, 6 analyses, a sample size of 354, 80% power and 2.5% (1-sided) type I error. The mortality rate in the control group was assumed to be 50%, and the new intervention could reduce the mortality rate by 15%. Fixed sample size with the same operating characteristics is 339. Efficacy bounds were derived using a Hwang-Shih-DeCani spending function with gamma =−4. Futility bounds were derived using a Hwang-Shih-DeCani spending function with gamma =−2 (16). Figure 1 shows the spending futility and efficacy boundaries where spending computations assume trial stops if a bound is crossed.
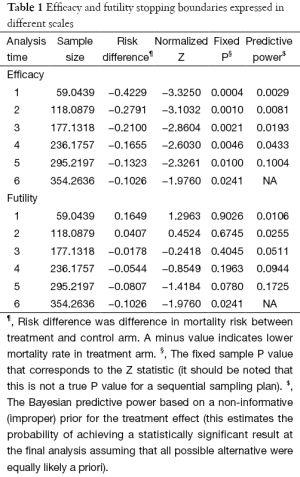
Full table
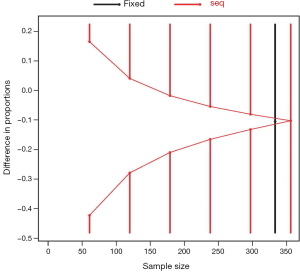
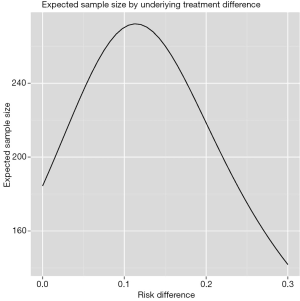
Figure 2 shows the cumulative probability of crossing boundary by different risk difference (effect size). Table 2 shows the average sample size and cumulative stopping probability at each analysis under alternatives detected with specified power. When the true effect is larger, it is more probable that the final required sample size will be smaller and the trial is more likely to stop early. Table 3 shows the inference at the stopping boundaries. The adjusted and unadjusted risk difference at each interim boundary is shown in Figure 3. Expected sample size varies by underlying different risk difference. It is shown that if the risk difference is extremely large or small the sample size required can be small. At the assumed risk difference of 0.15, the expected sample size is 260, which is smaller than the fixed sample size of 339 (Figure 4).
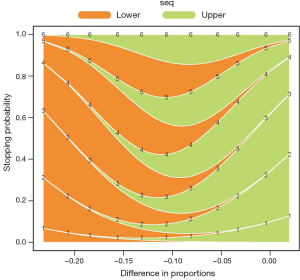
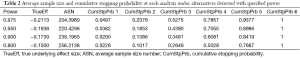
Full table
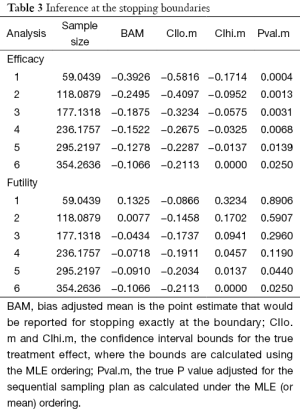
Full table
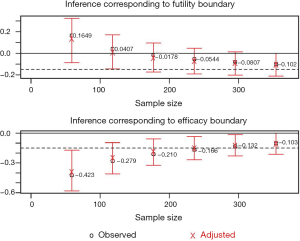
Statistical analysis
Baseline variables were expressed as mean (SD) or number (percent) as appropriate. Skewed data were expressed as median and interquartile range. Comparisons between treatment and control arms will be performed by using student t test for variables of normal distribution, Mann-Whitney U test for skewed variables. Categorical data were compared by using Chi-square test. Mortality is a binary variable and the comparison will be made by using Chi-square test. Post hoc analysis will be performed if there are differences on baseline variable between treatment and control arms. Furthermore, multivariable regression model will be used to control for potential confounders.
All statistical analyses will be performed by using the R software (version 3.1.1). Sequential trial analysis is performed by using the gsDesign and RCTdesign packages (17). Statistical significance will be considered at a P value of less than 0.05.
Discussion
The hallmark pathophysiology underlying septic shock is the dysfunction of microcirculation, following by tissue hypoperfusion, cell death and organ dysfunction (18,19). Up to now, varieties of strategies have been investigated for their potential effects on improving microcirculation. These strategies include but not limited to early fluid resuscitation to restore circulatory volume, use of vasopressors to maintain macrocirculation and use of some immunomodulatory agents (20,21). Although some treatments have once shown promising results, consequent large trials tempered such enthusiasm. As a result, treatment of septic shock remains a great challenge for clinicians.
Anisodamine is a muscarinic antagonist with pharmacological effects similar to atropine. It was first isolated from a traditional medicinal herb Scopolia tangutica Maxim in the middle of 1970s. This Chinese herbal medicine has long been used as an analgesic by local people in the regions of Qinghai and Xizang. Some animal studies have confirmed the role of anisodamine in improving microcirculation in septic shock, and other potential therapeutic effects include the inhibition of thromboxane synthesis, granulocyte and platelet aggregation (11,15). Although this drug is widely used in clinical practice in China, there is little clinical evidence from well-designed clinical trial that demonstrates its effectiveness in patients with septic shock. The present study was designed to bridge this gap.
One limitation of the study design is that the study is an open-labeled trial. However, we feel that this will not affect the primary outcome very much. The primary outcome used in the study is mortality that is solid and not prone to reporting bias. Other outcome assessors were blind to the allocation. Another limitation is that the titration of anisodamine infusion rate is largely determined by treating physicians. As a matter of fact, the therapeutic dose varied substantially among individuals and there is no rule-of-thumb for this titration. This is just like the titration of vasopressors. For example, the therapeutic dosage of norepinephrine ranges from 0.04 to 1 mcg/kg/min (22). Some patients may respond well to minimum doses, while others require large dose to maintain optimal MAP.
In conclusion, we believe that the study will provide new insight into the treatment of septic shock and can help to reduce mortality rate of septic shock.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhou J, Qian C, Zhao M, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS One 2014;9:e107181. [PubMed]
- Paulsen J, Mehl A, Askim Å, et al. Epidemiology and outcome of Staphylococcus aureus bloodstream infection and sepsis in a Norwegian county 1996-2011: an observational study. BMC Infect Dis 2015;15:116. [PubMed]
- Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [PubMed]
- Zhang Z, Ni H, Qian Z. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med 2015;41:444-51. [PubMed]
- Andrews B, Muchemwa L, Kelly P, et al. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia. Crit Care Med 2014;42:2315-24. [PubMed]
- Annane D, Aegerter P, Jars-Guincestre MC, et al. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med 2003;168:165-72. [PubMed]
- Gu WJ, Zhang Z, Bakker J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med 2015;41:1862-3. [PubMed]
- Zhang L, Zhu G, Han L, et al. Early goal-directed therapy in the management of severe sepsis or septic shock in adults: a meta-analysis of randomized controlled trials. BMC Med 2015;13:71. [PubMed]
- Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015;41:1549-60. [PubMed]
- Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015;372:1301-11. [PubMed]
- Zou AP, Parekh N, Steinhausen M. Effect of anisodamine on the microcirculation of the hydronephrotic kidney of rats. Int J Microcirc Clin Exp 1990;9:285-96. [PubMed]
- Poupko JM, Baskin SI, Moore E. The pharmacological properties of anisodamine. J Appl Toxicol 2007;27:116-21. [PubMed]
- Griffin RJ Jr. Herbal medicine revisited: science looks anew at ancient Chinese pharmacology. Am Pharm 1979;19:16-22. [PubMed]
- Anisodamine in treatment of some diseases with manifestations of acute microcirculatory insufficiency. Chin Med J (Engl) 1975;1:127-32. [PubMed]
- Xiu RJ, Hammerschmidt DE, Coppo PA, et al. Anisodamine inhibits thromboxane synthesis, granulocyte aggregation, and platelet aggregation. A possible mechanism for its efficacy in bacteremic shock. JAMA 1982;247:1458-60. [PubMed]
- Hwang IK, Shih WJ, De Cani JS. Group sequential designs using a family of type I error probability spending functions. Stat Med 1990;9:1439-45. [PubMed]
- Anderson K. gsDesign: Group Sequential Design [Internet]. [cited 2015 Aug 13]. Available online: https://cran.r-project.org/web/packages/gsDesign/gsDesign.pdf
- De Backer D, Donadello K, Cortes DO. Monitoring the microcirculation. J Clin Monit Comput 2012;26:361-6. [PubMed]
- Ratiani L, Gamkrelidze M, Khuchua E, et al. Altered microcirculation in septic shock. Georgian Med News 2015.16-24. [PubMed]
- Zhang Z. The efficacy of activated protein C for the treatment of sepsis: incorporating observational evidence with a Bayesian approach. BMJ Open 2015;5:e006524. [PubMed]
- van Haren FM, Sleigh J, Boerma EC, et al. Hypertonic fluid administration in patients with septic shock: a prospective randomized controlled pilot study. Shock 2012;37:268-75. [PubMed]
- Jentzer JC, Coons JC, Link CB, et al. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther 2015;20:249-60. [PubMed]


