Wound dressings for primary and revision total joint arthroplasty
Introduction
Wound management is an important part of preventing post-surgical complications after total joint arthroplasty (TJA). Application of an appropriate wound dressing is necessary for proper wound management. The purpose of applying a wound dressing is to provide mechanical protection to newly forming tissue, absorb exudate, stop bleeding and create a suitable environment for faster healing. Factors which strongly influence the choice of dressing after TJA currently include the surgeon’s familiarity and personal preference for a dressing, knowledge about dressings and the cost of the dressing.
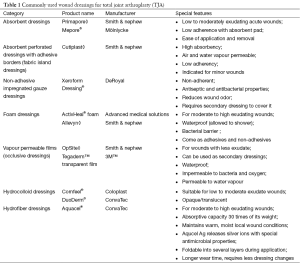
An ideal orthopaedic dressing should be: (I) absorbent; (II) protective; (III) cost effective; (IV) permeable; (V) transparent; (VI) able to provide a moist environment; (VII) able to remain in situ; (VIII) able to act as a complete barrier; and (IX) have low adherence (1).
To date, no TJA dressing encompasses all of the above parameters. However, there are many available wound dressings for TJA (Table 1). The purpose of this review is to address various wound dressings for primary and revision TJA, specifically looking at three main topics: (I) permeability and absorptive capacity of the dressing; (II) ability to decrease the risk of surgical site infection (SSI); and (III) cost effectiveness.
Full table
Permeability and absorptive capacity of dressing
Scientists have long believed that wounds should be kept dry in order to promote healing and formation of scar tissue. However, recent studies have demonstrated that moisture enhances the wound healing process and protects nerve endings from exposure and drying out. Wound exudate contains cells and growth factors that create the moist environment necessary for wound healing, but its accumulation can result in peri-wound blister formation. An ideal dressing should absorb the excess amount of exudate, but maintain a moist environment for wound healing (2).
To date, however, we still do not know exactly how much exudate a wound needs. As the wound heals further, the amount of exudate produced gradually decreases to zero. Maintenance of the ideal moist environment for TJA incisions depend upon two properties of the dressing: absorptive capacity and permeability.
Traditional cotton dressings have a high ability to absorb exudate. However, they dry out sooner and are unable to maintain a moist environment. Furthermore, there is the risk of pain and additional trauma when changing these dressings due to the growth of the granulation tissue into the dressing (3), which could possibly hinder the healing process. Fibers from these dressings are often shed into the wound, which can create a focal point for infection and may allow microbes to pass into the wound (1). However, the low price and simplicity of gauze dressings, along with high familiarity with the dressing, makes gauze dressings attractive to many surgeons.
Vapour-permeable film dressings allow the transmission of moisture; however, they have a very low absorptive capacity and often need to be changed when there is a moderate rise in the amount of wound exudate. Because of the increased exudate, there may be delayed wound healing, as demonstrated in a randomized controlled trial comparing occlusive dressings to gauze (4). The authors argued that gauze dressings effectively absorbed excess exudate from the wound and kept them free from bacterial contamination. However, vapour-permeable dressings are beneficial as they are impermeable to bacteria, whether used as secondary dressings or fabric island dressings (1).
To further prevent bacterial contamination, hydrofiber dressings, such as Aquacel® (ConvaTec, Greensboro, NC, USA), have been used in TJA. It has an absorptive capacity up to 30 times its weight, and it can keep exudate locked and away from the surrounding skin without losing integrity (5). This may lead to less blistering and epidermal stripping compared to traditional wound pads and tape or adhesive central pad dressings (1). Additionally, polymorphonuclear (PMN) leukocytes are often captured in the cross-linked hydrofiber network, and these activated granulocytes in the dressing exhibit antimicrobial action. A layer of fibrin is also formed between the wound bed and the dressing which provides a physical barrier to allow macrophages to heal the wound bed (6).
Another dressing type is hydrocolloid dressings. These dressings absorb exudate and form a gel that enhances the permeability of the dressing. This increases the loss of water in the form of water vapour and increases the capability of the dressing to cope with exudate production (7). The hypoxic and moist environment created by hydrocolloid dressings can improve wound healing. Additionally, these dressings lower the pH of the wounds to slightly acidic levels, which inhibits bacterial growth (1). This property of hydrocolloid dressings further prevents the likelihood of infection at the time of dressing changes when the wound is exposed to pathogens present in the environment.
Foam dressings are another type of dressing used after TJA. In a study by Thomas and Young (8), fluid handling properties were compared between two types of foam dressings: Allevyn◊ Adhesive (Smith & Nephew, London, UK), with an intelligent polyurethane film backing layer and ActivHeal® Foam Island (Advanced Medical Solutions, Cheshire, UK) with a standard backing film. The study revealed that the Allevyn◊ Adhesive was significantly more permeable than the ActivHeal® Foam Island, which could potentially reduce the number of dressing changes. However, both dressings were similar with regards to the rate of moisture vapour transmission and absorbency.
Reduction of SSI
SSI is a complication that can occur after TJA with reported postoperative infection rates in knee replacements ranging from 0.68% to 1.60% and from 0.67% to 2.4% in hip replacements (9).
Development of an SSI is often multifactorial in origin, and the difference in SSI rates due to wound dressings could possibly be explained by the following reasons: (I) number of dressing changes; (II) blister rate; and (III) skin injuries around the wound.
Number of dressing changes
Every time a dressing is changed, there is a potential risk for introducing pathogens into the wound, which can subsequently lead to SSI or periprosthetic joint infection (PJI). Wound dressings keep the wound near core body temperature, which increases the rate of miotic cell division and leukocyte activity that is necessary for wound healing. When a dressing is changed, it takes 3-4 hours for the cellular activity of the wound to resume. Hence, episodic cooling associated with dressing changes should be avoided as much as possible (1). Also, fewer dressing changes protects the wound from repeated exposure to pathogens in the surrounding air.
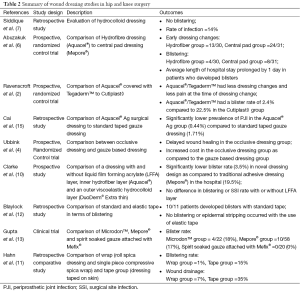
Abuzakuk et al. (6) demonstrated that there were less dressing changes for hydrofiber dressings within the first five postoperative days compared to the use of a central pad group. They theorized that leaving the hydrofiber dressing undisturbed for a longer period of time could help prevent wound infections.
Blister rate
Skin blistering is a common complication of wound dressings, and has been reported in as high as 13-35% of orthopaedic patients (2). Skin blistering occurs when the epidermis separates from the dermis secondary to continuous frictional forces on the skin. Usually, dressings are applied over the joint for a long period of time and may result in continuous shearing forces on the skin, eventually resulting in blister formation (2). Formation of blisters results in skin barrier breakdown and can increase the risk of developing a SSI (10). However, the dressing alone does not contribute to blistering (2). Various other factors that contribute to blistering include skin changes in older patients, soft tissue edema following surgery and the mode of dressing application. Dressings that are wrapped around a hip wound without tape demonstrate less blistering (1%) than dressings that are taped to the skin (15%) (11). The formation of blisters may also depend on the stretch of the dressing fibers. Blaylock et al. (12) found that the application of inelastic tape led to increased friction during movement and increased the blister rate, while elastic tape had a lower blistering rate. Another study by Gupta et al. (13) compared peri-wound blistering rates between a soft island dressing (Microdon™, 3M™, Saint Paul, MN, USA), Mepore® (Mölnlycke, Gothenburg, Sweden), and spirit-soaked gauze attached with Mefix® (Mölnlycke, Gothenburg, Sweden) and found that Microdon™ and Mepore® had more blistering than Mefix®. Applying the dressing fibers in the direction of joint movement reduced blister formation.
Blistering has been shown to be lower in certain advanced wound dressings because of their occlusive nature. A prospective, randomized control trial comparing Aquacel® covered with Tegaderm™ to Cutiplast◊ (Smith and Nephew, London, UK) showed a strong relationship between the type of wound dressing and its outcome (2). Aquacel® covered with Tegaderm™ was 5.8 times more likely to result in a wound with no complications and had less blistering (2.4%) compared to Cutiplast◊ (Smith and Nephew, London, UK) (22.5%). The lower rate of blistering with the hydrofiber dressing (Aquacel®) was due to vertical wicking, which significantly increases the volume of exudate that can be absorbed and reduces peri-wound blistering (2).
Hydrocolloid dressings, such as DuoDERM® (ConvaTec, Greensboro, NC, USA), have been shown to produce no blisters in hip and knee surgeries, but 14% of cases had serous discharge with no positive cultures (7). Combination dressings also demonstrate a low rate of blistering, as a dressing consisting of a liquid film forming acrylate (LFFA) layer applied to the periwound area, an inner hydrofiber layer (Aquacel®) and an outer viscoelastic hydrocolloid layer (DuoDERM®, Extra Thin, ConvaTec) had a 3.5% blistering rate (10). However, removal of the LFFA layer resulted in faster dressing application and decreased costs without affecting the blistering or SSI rate. Thus, the combination of hydrofiber and viscoelastic hydrocolloid in a dressing was adopted.
Skin injuries
Skin injuries around the wound can form a possible portal for bacterial entry into the surgical site. Studies have demonstrated that patients with superficial wound infections often present with a skin injury, but the causative role of skin injury in SSI has not yet been established (1).
Cost effectiveness
The costs associated with a wound dressing depends on two factors: (I) the unit cost of the dressing and (II) the number of dressing changes required. However, financial cost savings related to a dressing type depends on various factors, such as fewer post-operative wound complications such as blistering and SSI, shorter hospital stay, reduced number of dressing changes and less need for nursing care (14). Thus, while gauze with tape is the cheapest available dressing, it may not be the most cost-effective based on these other variables.
Cost analysis studies in TJA have compared hydrofiber dressing (Aquacel®) to a basic central pad, absorbent dressing (Mepore®), where dressings were left undisturbed on the wound for 5 days (6). Dressings were changed if it became soaked and/or the patient experienced discomfort. In the study, 13 out of 30 patients in the hydrofiber group had a dressing change within 5 post-operative days as compared to 24 out of 31 patients in the central pad group. Also, 4 out of 30 patients in the hydrofiber group developed blisters compared to 8 out of 31 patients in the central pad group. Almost twice as many patients developed blisters in the central pad group, which increased hospital length of stay by an average of 1 day and also increased patient discomfort (6). Therefore, the hydrofiber dressing which required fewer changes was cost-effective, required less nursing time and disturbed the wound less.
Another cost analysis study prospectively compared Aquacel® covered with Tegaderm™ (advanced fibrous hydrocolloid dressing) and Cutiplast◊ (absorbent perforated, wound contact dressing) in a randomized controlled trial (2). The end point of the study was a dressing failure or a wound that no longer needed any dressing change. The study found that patients receiving the Aquacel®/Tegaderm™ dressing needed fewer dressing changes, with less pain at the time of dressing change compared to Cutiplast◊. Although Aquacel®/Tegaderm™ is more expensive than Cutiplast◊, the authors argued that the extra wound dressing cost was compensated by fewer dressing changes and less wound complications.
A retrospective study by Cai et al. (15) compared acute PJI between Aquacel Ag® surgical dressing to a standard taped gauze dressing with Xeroform®. Acute PJI was significantly lower in the Aquacel® group (0.44%) compared to the standard dressing group (1.71%). While the cost of using Aquacel® dressing after TJA would be $27,000,000 anually, the fourfold reduction in PJI could save $375,000,000 (15).
Thus, these studies demonstrate that even though there is a higher unit cost associated with advanced wound dressings compared to standard wound dressings, these dressings can reduce the number of dressing changes, blistering and acute PJI.
Conclusions
The choice of TJA dressing type is often made by the operating surgeon in the operation room. Various factors contribute to the selection of a traditional dressing design despite the availability of newer designs. These include surgeon’s familiarity with a product, selection of the dressing by hospital administrators or staff, ease of application and availability of the dressing. However, for proper wound management, factors like permeability and absorptive capacity of the dressings, reduction in SSI, and cost of the dressing should play an important role in deciding the most suitable dressing type.
Wound dressings account for only about 0.02% of the total cost of a hip replacement, and a balance should be made between wound dressing expenditure and prevention of complications and wound dressing changes (1). Table 2 summarizes various studies on wound dressings in TJA. Based on the studies reviewed, hydrofiber and hydrocolloid dressings are recommended after TJA because they have high absorptive capacity and permeability, and can cope with exudate production. These dressings are changed less often and have low blistering rates, which may reduce SSIs. Although the unit cost associated with advanced dressings is much higher than traditional dressings, the decreased rate of PJI and costs associated with treating PJI more than compensate for it.
Full table
While this review provides a comprehensive evaluation of TJA dressings in literature, there are limitations to the studies included. The studies reviewed here are mostly retrospective and are often performed in small patient populations; thus, larger, multicenter studies are required for stronger evidence-based medicine. Additionally, wounds dressings should be applied according to the instruction manual, and improper application of wound dressings could result in incorrect outcomes (13). Finally, dressing changes depends upon the clinical judgement of the nursing staff and suffers from high subjectivity. Thus, the endpoint of wound dressing changes may not be ideal. Despite these limitations, this review provides an in-depth look at TJA dressings and provides orthopaedic surgeons with an overview of different dressing types and their mechanism of wound management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Collins A. Does the postoperative dressing regime affect wound healing after hip or knee arthroplasty? J Wound Care 2011;20:11-6. [PubMed]
- Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl 2006;88:18-22. [PubMed]
- Anderson I. Key principles involved in applying and removing wound dressings. Nurs Stand 2010;25:51-7. [PubMed]
- Ubbink DT, Vermeulen H, Goossens A, et al. Occlusive vs gauze dressings for local wound care in surgical patients: a randomized clinical trial. Arch Surg 2008;143:950-5. [PubMed]
- Williams C. An investigation of the benefits of Aquacel Hydrofibre wound dressing. Br J Nurs 1999;8:676-7, 680. [PubMed]
- Abuzakuk TM, Coward P, Shenava Y, et al. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J 2006;3:133-7. [PubMed]
- Siddique K, Mirza S, Housden P. Effectiveness of hydrocolloid dressing in postoperative hip and knee surgery: literature review and our experience. J Perioper Pract 2011;21:275-8. [PubMed]
- Thomas S, Young S. Exudate-handling mechanisms of two foam-film dressings. J Wound Care 2008;17:309-15. [PubMed]
- Greene LR. Guide to the elimination of orthopedic surgery surgical site infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology elimination guide. Am J Infect Control 2012;40:384-6. [PubMed]
- Clarke JV, Deakin AH, Dillon JM, et al. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care 2009;18:5-8, 10-1. [PubMed]
- Hahn GJ, Grant D, Bartke C, et al. Wound complications after hip surgery using a tapeless compressive support. Orthop Nurs 1999;18:43-9. [PubMed]
- Blaylock B, Murray M, O'Connell K, et al. Tape injury in the patient with total hip replacement. Orthop Nurs 1995;14:25-8. [PubMed]
- Gupta SK, Lee S, Moseley LG. Postoperative wound blistering: is there a link with dressing usage? J Wound Care 2002;11:271-3. [PubMed]
- Tustanowski J. Effect of dressing choice on outcomes after hip and knee arthroplasty: a literature review. J Wound Care 2009;18:449-50, 452, 454, passim. [PubMed]
- Cai J, Karam JA, Parvizi J, et al. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case-control study. J Arthroplasty 2014;29:1098-100. [PubMed]


