Somatostatin receptors over-expression in castration resistant prostate cancer detected by PET/CT: preliminary report of in six patients
Introduction
The meaning of neuroendocrine differentiation (NED) and somatostatin receptors (SSTRs) expression in castration resistant prostate cancer (CRPC) (1,2) has still not an established meaning (3-5). On the other hand, the possible presence of SSTRs on CRPC cell surface should provide a good therapeutic chance in patients with few treatment options to the oncologist. Serum chromogranin A (CgA) test has been the main surrogate parameter to define the SSTRs positivity. However, serum CgA raise in serum suffers of a low specificity (6-8), and moreover, it is not synonymous of SSTR expression (9). In summary, the raise of CgA does not guarantee the SSTR expression. Thus, a reliable and easy to use method to study in vivo SSTR presence should help significantly.
68Ga-DOTANOC is a radiopharmaceutical analog for SSTR2, SSTR3 and SSTR5, usually employed to image the neuroendocrine carcinomas (NET) with PET/CT. Aim of our phase IIIA trial to the study of CRPC is to detect their SSTRs overexpression by 68GA-DOTANOC PET/CT. Here we present the preliminary results of the first six patients recruited.
Material and methods
Six patients with CRPC were enrolled in this phase III trial (EUDRA CT number 2010-021026-35) granted by Regione Lombardia. The local Ethical Committee approved the trial design. All the patients gave their written informed consent before the enrollment in the study.
CRPC was defined a rise in serum prostate-specific antigen (PSA) and/or progression of pre-existing disease and/or appearance of new metastases despite androgen deprivation therapy (ADT). Basically, our recruitment criteria encompassed: (I) asymptomatic non-metastatic CRPC; (II) asymptomatic metastatic CRPC with prior treatments; (III) symptomatic, metastatic CRPC with prior treatments. For the first group of patients, the PSA was in unremitting raise for more than three consecutive evaluations during ADT. No measurable lesions, KPS >80%, life expectancy >3 months, good hematologic parameters and a wash-out time from the last chemotherapy of at least one month were requested. The main exclusion criterion was age (less than 18 and more than 85 years old).
One week after the patients were declared eligible for the trial, they were admitted to our hospital.
PET/CT was carried one hour after the i.v. administration of nearly 185 MBq of 68Ga-DOTANOC, synthesized following the procedures reported in the literature (10). PET/CT scan was carried out with a Siemens Biograph 6 PET/CT scanner (Siemens Healthcare, Italia), and the acquisition parameters for the CT were: kV =130; effective mAs =70; maximum reconstructed width =5 mm without overlap; pitch 1.5 mm; standard reconstruction algorithm. PET was performed from the lower thighs, with 6 bed positions (3 min per bed) and reconstructed using standard algorithms provided by Siemens.
Following our trial’s specifications, only one 68Ga-DOTANOC PET/CT examination was carried out in these patients.
Results
The main clinical characteristics of the six patients at diagnosis of PC are summarized in Table 1. The main clinical characteristics of the six patients at the onset of castration resistance are summarized in Table 2.
Full table

Full table
The five patients with bone metastases had diffuse multiple localization patterns at CT and bone scan. One of these had also an impressive lung involvement with lymphangitic carcinomatosis at CT scan.
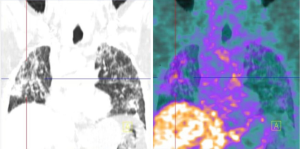
68Ga-DOTANOC PET/CT was positive in two patients. In patient 5 some areas of uptake were detected in both lungs in areas of irregular septal thickening, consistent with the lymphangitic spread (Figure 1) and in bone metastases previously evidenced by CT scan.
The second patient positive to 68Ga-DOTANOC PET/CT (patient 1) had multiple bone metastases detected at the diagnosis carried out one year before. The examination showed multiple areas of radiopharmaceutical’s uptake (Figure 2).
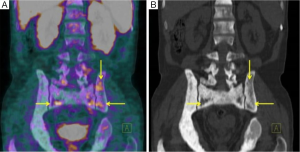
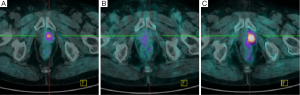
Figure 3 shows the negative pattern of 68Ga-DOTANOC uptake in patient 3, and compared it with the findings of 18F-Choline PET/CT. This patient was the only patient without already known parenchymal metastases at the enrollment. His recent pathological anamnesis clued of a hormonal recurrence. 18F-Choline PET/CT carried out to restage him detected a focal uptake in the left side of the prostate (Figure 3A). However, this finding was not considered diagnostic due to the occasional and unpredictable uptake of the radiopharmaceutical in non prostatectomized patients, Thus, the nodule was not studied by biopsy and a wait and see strategy was decided. Two months later the patient was recruited due to the continuous raise of PSA in spite of ADT. 68Ga-DOTANOC PET/CT did not disclose any uptake in the whole of body. More in detail, no specific uptake in the area corresponding to the previous 18F-Choline PET/CT was detected (Figure 3B). One more time, a wait and see strategy was adopted. The 18F-Choline PET/CT carried out four months later, confirmed and reinforced the finding of the first similar examination showing an increased uptake in the left side of the prostate gland (Figure 3C). This final evidence was considered suffices for the diagnosis, no biopsy was decided and the patient started abiraterone acetate treatment. Therefore, in this case 68Ga-DOTANOC PET/CT did not disclosed SSTRs in the relapse.
Discussion
The presence of SSTR in PC is still not completely understood. Some studies suggests that SSTR2 are overexpressed in CRPC (11,12) whereas others disagree with this finding (13-15). These contradictory results perhaps reflect the pattern of receptor expression, which are probably different in the primary compared to the metastatic disease.
The main incentive to study and quantify SSTRs in CRPC is to evaluate the possibility to use them as a therapeutic target with somatostatin analogs. Therefore, we did not pursue a diagnostic objective i.e., we did not look for metastases. Indeed, their presence was one of the inclusion criteria of our trial. In this regard, the terms true positive or false positive lose their emphasis. Indeed, of the pivotal point of our examination shifted from the detection of possible, but still not proved, metastases (typical of the “pure” diagnostic approach) towards the description of the receptorial panel of the widespread secondary. In conclusion, the question to answer with 68Ga-DOTANOC is: do these neoplasms overexpress SSTRs? And in how many of the metastases show significant SSTRs expression? Could this information be worth for therapeutic purposes?
Some researchers treated patients affected by CRPC on the base of serum CgA level taken as a surrogate marker of SSTRs expressions. However, serum CgA elevation is not a synonymous of this biological behavior (16-18). A possible comment is this approach, “blind” to the effective expression of the receptors, may partly explain the poor response to somatostatin analogs.
Nuclear Medicine procedures with gamma emitting radiopharmaceuticals have been occasionally employed in the past to detect SSTR overexpression in PC (19,20). In the last few years, newer PET/CT radiopharmaceuticals have been synthesized. These are 68Ga-DOTATOC, 68Ga-DOTANOC and 68Ga-DOTATATE, three almost similar compounds with only slight differences in chemical structure and receptor’s affinity. In 2010, the first study to evidence SSTRs overexpression in CRPC with 68Ga-DOTATOC, revealed a weak uptake of the metastases. The researcher concluded suggesting the use of a radiopharmaceutical with different affinity for SSTRs (21). 68Ga-DOTANOC differs with 68Ga-DOTATOC in the amino acidic sequences. This change results in different receptors affinity, i.e., 68GA-DOTANOC binds to SSTR types 2, 3 and 5, whereas 68Ga-DOTATOC lacks of affinity for SSTR3. Thus, if some neoplasms overexpress SSTR3 in a significant quota, they may be imaged with 68Ga-DOTANOC but not by 68Ga-DOTATOC.
Our case series showed 68Ga-DOTANOC uptake in two patients with skeletal and in lung metastases. The possible criticism is that the real correspondence between SSTRs expression evidenced by 68GA-DOTANOC and real presence of SSTRs on cell surface may be reached only with tissue samples. In our opinion, however, the clinical, radiologic and biochemical scenario of our patients give strong evidences about the metastatic nature of skeletal and pulmonary changes. Moreover, the proposal to biopsy a suspected skeletal metastasis in a plural-treated patient in sharp clinical, laboratory and radiological disease progression should be criticized from the ethical point of view. Indeed, a biopsy to test SSTRs expression could be justified only if it results in a variation of the treatment, which is the aim of our study. The same criticism could hamper the definition of a control population. Our regulatory rules are intransigent in avoiding unnecessary radiation exposure in patients and healthy population. However, the experience of 68GA-DOTANOC biodistribution in patients affected by in neuroendocrine tumors provided us useful information to define that skeletal and lung uptakes were abnormal. It goes without saying that the study of organs involved in 68Ga-DOTANOC clearance (liver, kidneys) or physiologic uptake (spleen) is not possible. On the other hand, these anatomic districts are not preferential sites of CRPC metastatization. Finally, it must be stressed that unlike 18F-FDG, 68Ga-DOTANOC is not a “metabolic” tracer. Thus, its uptake is far more dependent by SSTRs overexpression than by the blood flow.
Unfortunately, 68GA-DOTANOC uptake in CRPC (SUV mean 1.57) is scant if compared to neuroendocrine tumors. Probably this finding comes from they are not naïve neuroendocrine neoplasms thus process to express receptors is not fully accomplished.
We are unable to assess the prognostic significance of SSTRs overexpression. Surely, the paucity of their number hampers the use of similar radiolabeled compounds for treatment, as it comes for neuroendocrine tumors. Indeed, this kind of treatment calls for a higher tumor to background ratio to balance renal and hematological toxicity. Nevertheless, the clinical relevance of SSTRs overexpression should not be unacknowledged, particularly in those patients in which a significant amount of receptor is detected. The hypothesis to add somatostatin analogs to the usual therapeutic schedules as a complement to other pharmaceuticals could be considered especially in light of its low toxicity. The hart of the cultural leap is to start thinking this examination like a bridge between the diagnosis and therapy. In this setting, 68GA-DOTANOC PET/CT may play a pivotal role.
Acknowledgements
Thanks Mrs. Gabriella Taddeo for her grammar review.
Funding: Regione Lombardia “Call for Independent Research 2009”.
Disclosure: The authors declare no conflict of interest.
References
- Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148-59. [PubMed]
- Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA guideline. Journal of Urology 2013;190:429-38. [PubMed]
- Cohen MK, Arber DA, Coffield KS, et al. Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer 1994;74:1899-903. [PubMed]
- Deng X, Elzey BD, Poulson JM, et al. Ionizing radiation induces neuroendocrine differentiation of prostate cancer cells in vitro, in vivo and in prostate cancer patients. Am J Cancer Res 2011;1:834-44. [PubMed]
- Marchiani S, Tamburrino L, Nesi G, et al. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl 2010;33:784-93. [PubMed]
- Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol 2007;25:1967-73. [PubMed]
- Lawrence B, Gustafsson BI, Kidd M, et al. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:111-34. [PubMed]
- Bech PR, Ramachandran R, Dhillo WS, et al. Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine- and amphetamine-regulated transcript. Clin Chem 2012;58:941-3. [PubMed]
- Matei DV, Renne G, Pimentel M, et al. Neuroendocrine differentiation in castration-resistant prostate cancer: a systematic diagnostic attempt. Clin Genitourin Cancer 2012;10:164-73. [PubMed]
- Pettinato C, Sarnelli A, Di Donna M, et al. 68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2008;35:72-9. [PubMed]
- Hansson J, Bjartell A, Gadaleanu V, et al. Expression of somatostatin receptor subtypes 2 and 4 in human benign prostatic hyperplasia and prostatic cancer. Prostate 2002;53:50-9. [PubMed]
- Morichetti D, Mazzucchelli R, Stramazzotti D, et al. Immunohistochemical expression of somatostatin receptor subtypes in prostate tissue from cystoprostatectomies with incidental prostate cancer. BJU Int 2010;106:1072-80. [PubMed]
- Halmos G, Schally AV, Sun B, et al. High expression of somatostatin receptors and messenger ribonucleic acid for its receptor subtypes in organ-confined and locally advanced human prostate cancers. J Clin Endocrinol Metab 2000;85:2564-71. [PubMed]
- Cariaga-Martinez AE, Lorenzati MA, Riera MA, et al. Tumoral prostate shows different expression pattern of somatostatin receptor 2 (SSTR2) and phosphotyrosine phosphatase SHP-1 (PTPN6) according to tumor progression. Adv Urol 2009;723831. [PubMed]
- Hennigs JK, Müller J, Adam M, et al. Loss of somatostatin receptor subtype 2 in prostate cancer is linked to an aggressive cancer phenotype, high tumor cell proliferation and predicts early metastatic and biochemical relapse. PLoS One 2014;9:e100469. [PubMed]
- Schilling D, Küfer R, Kruck S, et al. Somatostatin analogs for the treatment of advanced, hormone-refractory prostate cancer: a possibility for secondary hormonal ablation? Urologe A 2008;47:1334-8. [PubMed]
- Friedlander TW, Weinberg VK, Small EJ, et al. Effect of the somatostatin analog octreotide acetate on circulating insulin-like growth factor-1 and related peptides in patients with non-metastatic castration-resistant prostate cancer: results of a phase II study. Urol Oncol 2012;30:408-14. [PubMed]
- González-Barcena D, Schally AV, Vadillo-Buenfil M, et al. Response of patients with advanced prostatic cancer to administration of somatostatin analog RC-160 (vapreotide) at the time of relapse. Prostate. 2003;56:183-91. [PubMed]
- Thakur ML, Kolan H, Li J, et al. Radiolabeled somatostatin analogs in prostate cancer. Nucl Med Biol 1997;24:105-13. [PubMed]
- Kälkner KM, Nilsson S, Westlin JE. [111In-DTPA-D-Phe1]-octreotide scintigraphy in patients with hormone-refractory prostatic adenocarcinoma can predict therapy outcome with octreotide treatment: a pilot study. Anticancer Res 1998;18:513-6. [PubMed]
- Luboldt W, Zöphel K, Wunderlich G, et al. Visualization of somatostatin receptors in prostate cancer and its bone metastases with Ga-68-DOTATOC PET/CT. Mol Imaging Biol 2010;12:78-84. [PubMed]


