Non-intubated video-assisted thoracic surgery management of secondary spontaneous pneumothorax
Authors’ introduction:
Figure 1 is part of our team at the University General Hospital of Alicante, and it is composed by thoracic surgeons (Dr. C. Galvez, Dr. S. Bolufer), anesthesiologists (Dr. J. Navarro-Martinez, Dr. MJ. Rivera, Dr. M. Galiana) and the nurses (Mrs. M. Perez, Mrs. E. Ortuño, Mrs. F. Rey). We began on July 2013 with non-intubated uniportal VATS procedures and we are evolving in the anesthetic care, major procedures and fast-track protocols to achieve the least invasive approach for our patients.

Introduction
A pneumothorax is defined as an abnormal accumulation of air in the pleural space, and we call it spontaneous in the absence of thoracic trauma. In contradistinction to primary spontaneous pneumothorax (PSP) which usually occurs in young healthy males who do not have clinically apparent lung disorders, secondary spontaneous pneumothorax (SSP) is associated with underlying pleural or lung disease, such as chronic obstructive pulmonary disease (COPD) in most cases (Figure 2), cystic fibrosis, interstitial lung disease (ILD) or others, and has greater consequences (higher morbidity and mortality) and more challenging management due to the more compromised pulmonary status of these patients, comparing to PSP (1). COPD is the more frequent cause of SSP, and the probability of developing an episode increases proportionally to the deterioration of FEV1 value and FEV1 to forced vital capacity (FVC) ratio (2). The global incidence of SSP has been reported as 3.8/100,000, being more likely in men (6.3/100,000), than in women (2/100,000), with a peak incidence at 60-65 years of age. The recurrence rate reaches the 40-56% of all first episodes, with age, pulmonary fibrosis and emphysema as risk factors for recurrence, and can occasionally be lethal (3) increasing the mortality by 3.5 times with each pneumothorax occurrence in COPD (4). All the SSP patients should be admitted to hospital, and most of them need a chest drain, trying to be as conservative as possible in most cases. In those cases of intractable pneumothoraces, or in the case of prolonged air leak, surgical treatment with video-assisted thoracic surgery (VATS) for bullectomy and pleurodesis has shown good results, generally performed under GA and lung isolation with double-lumen endotracheal tube (5). Despite its extended use in many different surgeries, risks of GA and mechanical ventilation (MV) are not negligible, especially in patients with poor performance status (PS) and cardiorespiratory disease. Impaired cardiac function, haemodynamic instability, alveolar barotrauma, volutrauma and atelectrauma with increased risk of pneumonia are described, and also neuromuscular blockade increases atelectasis in the non-operative dependent lung with right-to-left shunt and increased risk of hypoxia and ventilator dependency (6). These risks shouldn’t be underestimated in patients with SSP.
Awake procedures in non-intubated patients, avoid GA and ventilator-induced damages, and have shown encouraging results in many surgical procedures such as pulmonary wedge and anatomical resections, pleural effusions, hyperhidrosis, thymectomies and lung-volume reduction surgery (LVRS) (7). In the last years, some groups have reported their results with different approaches under local or regional anesthesia in the treatment of primary and SSP.
The aim of this review is to collect all the experience of different non-intubated VATS (NI-VATS) strategies with local and/or epidural thoracic anesthesia for SSP and analyze their results.
Adverse effects of GA and MV in pulmonary compromised patients
GA is nowadays the first-line gold standard anesthetic strategy in thoracic surgery with single-lung MV. It can be performed with double-lumen tube (DLT) orotracheal intubation or with endobronchial blockers (EB). With the smaller internal diameters of DLTs, plateau pressure and peak inspiratory pressure increase in a 42% and 55% respectively, with the inherent risk of lung damage with high plateau pressures (8). The loss of diaphragm motion with muscle relaxation and displacement by abdominal organs, the loss of intrapleural negative pressure in the operative lung with mediastinal weight release onto the lower lung, and the decrease of functional residual capacity (FRC) of the dependent non-operative lung, which is perfusion favored during lateral decubitus, originates an important disturbance in the ventilation/perfusion (V/Q) matching. With ventilation restriction to the non-operative lung, the perfusion through the operative lung increases the physiological right-to-left shunt, leading to potential intraoperative hypoxemia. The physiological mechanism of hypoxic pulmonary vasoconstriction (HPV), which closes blood vessels in the non-ventilated areas thus redirecting the flow to ventilated areas, protects from this shunt. Volatile anesthetics used in GA have been reported to inhibit this mechanism, whilst other intravenous agents like propofol and thoracic epidural agents have little or no effect on HPV. This shunt fraction can increase if atelectasis develops in the non-operative lung, due to extrinsic compression by mediastinum, abdominal organs, and low tidal volumes of protective lung ventilation protocols (9). Oxygen toxicity due to ischemia-reperfusion injury and oxidative stress is another consequence of single-lung ventilation, and should be minimized with the lowest FiO2 possible (10), and the rational use of PEEP for improving oxygenation trying to avoid excessive lung overdistention. Besides this, MV can produce barotrauma (airway pressure-induced injury), volutrauma (lung inflation-induced injury), atelectrauma (opening-closing cycle) and biological trauma (proinflammatory mediators release), increasing morbidity and mortality. All these deleterious effects of GA and MV should be highlighted in patients with SSP and underlying pulmonary disease because they can fatally influence the postoperative course of the patients, although there is still a paucity of studies assessing the effects of GA in this group of patients.
There are not too many reports of surgery in SSP, but it seems logical that patients with SSP are at a high risk of developing postoperative complications due to their worsened general status and their underlying pulmonary disease, with a postoperative morbidity rate between 15-27.7% in a recent review (11). Many factors like prolonged air leak, elevated PaCO2 level, or severity of the pulmonary disease have been correlated with increased postoperative complications in these patients (12).
Thus, although considered the gold standard for the anesthetic management of thoracic surgery patients, and in spite of being a benign disease, the potential risks of SSP surgery under GA and MV should be taken into account. The scenario in SSP shows to be different from that of PSP in young patients without evidence of pulmonary disease, so searching for new less invasive and aggressive anesthetic strategies becomes essential.
Potential advantages of locoregional anesthesia in awake non-intubated patients with SSP
First of all, keeping diaphragm motion during awake procedures avoiding muscle relaxation (Figure 3), preserves the compliance in the dependent non-operative lung, which in addition to a better perfusion by gravity minimizes the disruption in the match of V/Q, compared to GA (14), thus decreasing the risk of hypoxemia in patients with pulmonary disease.

Intravenous anesthetics like propofol, and thoracic epidural agents (bupivacaine, ropivacaine, xylocaine) have shown to affect little or nothing the HPV mechanism, thus reducing the risks of hypoxemia due to right-to-left shunt (15,16). This proves to be safer when dealing with patients with chronic drop of oxygen saturation like severe emphysema or pulmonary fibrosis.
Some meta-analysis showed that thoracic epidural anesthesia (TEA) may potentially decrease cardiac morbidity and mortality after non-cardiac procedures (17,18). Other meta-analysis have shown that epidural anesthesia reduces postoperative morbidity and mortality (19,20) comparing to GA, thus decreasing pulmonary infections to about a third, and overall pulmonary complications to about a half comparing to GA (19). The explanation for this probably relies on earlier mobilization of the patients, adequate pain control for coughing and reduced opiod consumption (21) lead to reduce the risk of secretions retention, atelectasis and pulmonary infection. Of course, avoiding orotracheal intubation and MV seems to reduce postoperative complications such as pulmonary infections, as the traumatic damage to the bronchial and tracheal mucosa is avoided thus diminishing airway inflammation and manipulation of the non-sterile tracheobronchial tree.
Oxygen saturation over 90% can be kept in these spontaneously breathing patients with oxygen support delivered with nasal prongs, facial mask, high-flow oxygen devices or intranasal multi-perforated cannula above the larynx (Figure 4). We should be very cautious with sedation because it favors hypercapnia, and in patients with chronic high levels of PaCO2 should be kept in mind. The permissive hypercapnia is very common in non-intubated patients, but typically is well tolerated. Monitoring directly pH, PCO2 and PO2 through the invasive monitorization using the radial artery is important. In case severe hypercapnia develops, a non-invasive ventilator should be prepared. It is better to avoid sedation as much as possible, letting the patient to communicate with the anesthetic team thus increasing or decreasing depth of ventilation depending on the respiratory parameters and the need of both the surgeon and the anesthesiologist.

NI-VATS with TEA or combined with local anesthesia for SSP
There are not too many reports of NI-VATS procedures under TEA or combined with TEA plus local anesthesia in patients with secondary pneumothorax, and there is a complete lack of clinical trials, so the evidence level reaches at most a level 2- (cohort or case/control studies with high bias risk) or level 3 (non-analytic studies like case reports and case series). This poorness of trials impedes to settle strong recommendations, which are limited to a D degree of the Scottish Intercollegiate Guidelines Network (SIGN) (22). It is important to keep in mind that serious ethical considerations arise when dealing with patients with moderate to severe pulmonary disease if we consider to randomize into two groups with TEA ± local anesthesia or GA in the light of a high respiratory complication rate and great risk for ventilator dependency (6).
There is only one comparative retrospective study, published by Noda et al. in 2012 (23). They performed during a 5-year period 57 VATS on SSP patients with air leak of more than 7 days. A total of 42 patients were operated with the standard GA and orotracheal intubation, and 15 underwent surgery with TEA and/or local regimen. There were no specific criteria for selecting patients for the TEA/local arm, but it was applied in patients with poorer PS and more oxygen requirement (P=0.009 and P=0.0002 respectively). With a propensity score matching analysis to minimize differences between the groups, comparable groups of eight patients each with almost identical characteristics were found and there were no statistical differences between them. All procedures in the TEA arm were performed with a catheter at T3-6 level instillating ropivacaine 0.2% in continuous infusion, with lidocaine 1% in the 2-3 ports. Oxygen supplement was administered to keep oxygen saturation above 94%, but there is no explanation about the oxygen delivery system. A Roeder loop and endostaplers were used for treating primary lesions, and no systematical pleurodesis was performed during the surgery. They found an important decrease in the operating room (OR) time in the TEA arm (116.5±5 vs. 209.1±77.1 minutes) which proved to be significant in the entire study and the propensity-score matched pairs (P<0.0001 and P=0.006). There were four cases of prolonged air leak (>7 days) in the TEA arm for three cases in the GA arm (P=0.05) but significance disappeared after the propensity-score analysis (P=0.60). Postoperative respiratory complications [pneumonia and acute respiratory distress syndrome (ARDS)] happened during GA in ten cases, but none in the TEA arm (P=0.046) and this difference was kept significant in the propensity analysis (P=0.02). Hospital stay was not significantly different and four hospital deaths were observed in the GA arm (pneumonia in two cases and ARDS in other two cases), without reaching the statistical significance (P=0.25). Authors concluded that TEA could decrease total OR time and postoperative respiratory morbidity, even in more compromised patients, hypothesizing that avoiding barotrauma and the risk for aspiration could benefit this group. This approach proves to be feasible and with an acceptable pattern of morbidity, but additional studies are needed, stated the authors.
There are two case series assessing non-intubated epidural regimens for SSP. Mukaida et al. in 1998 (24) reported four cases of intractable SSP with a drainage duration between 22 and 49 days preoperative. All patients were performed a pleurography, but there are no details. Epidural catheter with bupivacaine 0.26%, intravenous sedation with fentanyl and local 1% lidocaine were administered for a 3-port VATS approach and oxygen supplementation with facial mask. Fibrin glue and polyglycolic acid sheet were used to treat the pulmonary leak. There were no hemodynamic disturbances during surgery, and no postoperative complications were reported. There was no recurrence during a 16-month follow-up, so they conclude that TEA NI-VATS surgery for SSP is a safe procedure, and that the fibrin glue and polyglycolic acid sheet technique can control the leak in these patients.
Kiss and colleagues (6) reported in 2014 an observational retrospective series of nine cases of severely dyspnoeic patients excluded from GA, needing thoracic surgery (thoracotomy in six cases and VATS in three cases), five of them with intractable pneumothorax. Different underlying pathologies were registered: idiopathic pulmonary fibrosis in two cases, multiple bilateral pulmonary metastasis in three cases, severe COPD in one case, inflammatory alveolitis in one case, and advanced myopathy in two cases. All patients were high risk of the American Society of Anesthesiologist (ASA 4), and 8 were also grade 4 of the Modified Medical Research Council (MMRC) dyspnea scale, so this is the first report of TEA in which ASA and dyspnea scale are mentioned. Six of the nine patients needed oxygen preoperatively. TEA catheter was inserted between T3-7 levels with lidocaine 20 mg/mL, ropivacaine 7.5 mg/mL, and/or xylocaine 1-2%, and none or only mild sedation with propofol and remifentanyl or only remifentanyl was administered in order to avoid hypoventilation and hypercapnia. High flow oxygen with ventimask at 15 L/min was needed in all the patients to achieve oxygen saturation between 85-100%, and only in one case of severe COPD 2 L/min was administered, being saturation during surgery between 85-97% all the whole procedure. One of the patients with advanced myopathy even needed during surgery her own BiPAP ventilation routine level. All patients received PEEP of 5 cmH2O for lung re-expansion, and non-invasive ventilation after the surgery to prevent atelectasis in the absence of air leak. Surgical time was 76 minutes and total OR time of 170 minutes, with no need for conversion to GA. Respiratory complications during surgery were only one hypercapnia with 79 mmHg end-tidal CO2 (ETCO2), and one perioperative hypercapnic coma (ETCO2 58 mmHg), both of them managed with assisted mask ventilation in the spontaneously breathing patient. All patients resume oral intake at postoperative day (POD) 1, and there was no complication related to TEA. One patient with idiopathic pulmonary fibrosis developed ADRS, dying at POD 25. One patient died at home 2 months after the surgery and postoperative chemotherapy, and seven patients were still alive at 6 months after the procedure. All patients were satisfied with TEA anesthesia. The limitations of the study are its non-randomized and retrospective design, and the small sample of patients. Ethical considerations and the low prevalence of this severely dyspnoeic patients which can improve with surgery are the explanations of the authors. They also make no monitoring of PaO2, but oxygen saturation was kept in all patients between 85-100%. There was also no specific protocol with TEA, so it was left to the discretion of the anesthesiologist.
In 2005, Sugimoto et al. (25) described their experience with two cases of pneumothorax in the native lung after lung transplantation. The risk of pneumothorax is not negligible secondary to bronchial anastomosis stricture thus leading to preferential ventilation in the native one, progression of the underlying disease (emphysema, pulmonary fibrosis, lymphangioleiomyomatosis), or prolonged MV in case of living-donor lobar lung transplantation. They describe a technique with TEA plus local anesthesia in a multiportal VATS approach, controlling the air leak with fibrin glue and polyglycolic acid sheet, with excellent results and no recurrence in a 40 and 23 months follow-up respectively. The potential benefit of non-intubated procedures in these two cases relies on avoiding deleterious effects of MV through a bronchial anastomosis stenosis and a small graft of one lobe.
On the other hand Noda et al. (26) reported a successful management of a right pneumothorax in a patient with a previous left pneumonectomy for non-small cell lung cancer (NSCLC), with emphysema and bullas in the right lung. A TEA catheter with ropivacaine 0.2% plus local infiltration of lidocaine 1% were used in a 2-port VATS, resecting the bulla with an endostapler and polyglycolic acid sheets, and spraying fibrin glue over the suture. Oxygen supplement was administered in order to keep saturation above 95% during all the procedure. Patient was discharged on POD 4 and there was no recurrence, concluding that it constitutes a safe strategy for managing pneumothorax in patients with a contralateral pneumonectomy and underlying disease in the affected lung.
We cannot finish this review without mentioning the valuable input of Dr. Pompeo, Mineo and their Awake Thoracic Surgery Research group in two Letters to Editor published in 2012 (27,28). Given their worldwide known experience with awake thoracic surgery procedures, and in response to the report of Noda above mentioned (23), they showed their initial results with 23 SSP. In their series, surgical time between 35 and 90 minutes, and mean hospital stay of 3 days were reported. In a follow-up between 12 and 108 months they had no recurrence of pneumothorax. They also highlighted that mean drop in PaO2 to inspired fraction of oxygen ratio was markedly lower in patients with underlying severe COPD (34 vs. 77 mmHg in normal lungs), and they correlate this with severe trapping of air in COPD patients, which can counteract the effect of atmospheric pressure over the operating lung, due to a PEEP effect and elongation of exhalatory time. They also proved that awake procedures in SSP attenuated release of stress hormones (cortisol), inflammation markers (IL-6) and natural killer cells activation, hypothetizing that, as it preserves more the immune response, could potentially explain the decrease in postoperative infections.
NI-VATS with local anesthesia for SSP
When we analyze the works concerning NI-VATS surgery for SSP performed under local anesthesia with or without sedation, we find only one comparative study, one case series, one case-report and one letter to editor. The lack of scientific evidence is clear and there is a complete lack of clinical trials, so the evidence level reaches at most a level 2- (cohort or case/control studies with high bias risk) or level 3 (non-analytic studies like case reports and case series). It is not possible to settle strong recommendations, which are limited to a D degree of the SIGN (22).
In 1997, Nezu et al. (29) published what constitutes the earliest comparative study of NI-VATS surgery for pneumothorax including SSP ever published. They reported 34 cases of persistent or recurrent pneumothorax (primary and secondary) operated on with local anesthesia and sedation. They compared with 38 patients operated under GA, but there are no specified criteria for selecting patients for one or the other regimen, although all the patients in the local group had no adhesions and radiologically localized bullas previous to the operation. They included both primary (PSP) and secondary pneumothorax (SSP). Lidocaine 0.5% in the pleural and intercostal space, and butorphanol tartrate and intravenous diazepam were used to achieve local somatosensory block and mild sedation. With 3-port VATS they performed resection of the bulla with endostapler and fibrin glue aerosolized. Overall success rate was achieved in 94% of the patients, with two cases needing a later open procedure under GA because of anxiety attack and severe adhesions. Respiratory and hemodynamical parameters kept into normal values, with 44.6 minutes of mean surgical time and 4.5 days of mean length of stay. There were three postoperative complications (9.4%): one case of atelectasis solved with conservative treatment, and 2 prolonged air leaks, one of them solved with minocycline pleurodesis and the other stopped spontaneously on 6th day. Only one patient had a recurrence (3.2%), and needed a new surgical procedure. They found statistical differences in surgical time and length of stay favoring patients operated with local anesthesia against GA (44.6±11.6 vs. 63.3±20 minutes, P<0.01; 4.5±1.3 vs. 5.8±1.1 days, P<0.01).
The same year, Tschopp et al. (30) described their results with a series of 89 patients with recurrent or secondary pneumothorax, where 93 toracoscopies (2-port) and talc pleurodesis with local anesthesia (lidocaine 1%) were performed. For premedicating these patients they used atropine 0.5 mg, midazolam 3-10 mg and petidine 25-100 mg. They didn’t resect any lung parenchyma nor any bulla, just applied 3 to 5 mL of talc all over the pleural surfaces. Mean drainage time of 5 days and mean length of stay of 5.2 days were reported. Only mild postoperative complications were described (subcutaneous emphysema, arrhythmia, pneumonia and bronchospasm). Nine patients needed a second procedure, where seven succeeded repeating the same operation, and two needed VATS or thoracotomy. There were six recurrences (6.4%), where three of them succeeded repeating the procedure, two spontaneously and one needed a later thoracotomy. They found bulla size bigger than 2 cm a risk factor for recurrence (40%). They concluded that this approach was safe, cheap and simple for the treatment of complicated pneumothorax.
In 1999, de la Torre Bravos et al. (31) in a letter to editor mentioned their experience with two cases treated with local mepivacaine 200 mg and intravenous midazolam 5 mg, with successful results.
Finally, in 2012 Yutaka et al. (32) published a case of persistent SSP treated under local anesthesia (lidocaine 1%) with a VATS approach, just using polyglycolic acid sheet, fibrin glue and autologous blood on the air leak. Operative time was 55 minutes and drainage time 5 days and there was no recurrence.
Discussion
The review of scientific evidence about the experience with NI-VATS in the management of SSP, must focus in three main aspects: first of all, what we expect about potential benefits of this less-invasive anesthetic/surgical approach in these deteriorated patients; secondly, the results of the little experience collected through the last two decades, mainly case series and some comparative study; third of all, which ones should be the lines for further research and investigation.
The first issue focuses on elucidating the potential advantages that would make a non-intubated VATS approach better than the conventional one with GA and MV. Our current knowledge about pathophysiology of surgical pneumothorax in a spontaneously breathing patient evidences that it produces both positive and negative effects in the respiratory balance of the patient (33). On the one hand, preserving the diaphragm motion, conserves the pulmonary compliance of the non-operative lung, which is perfusion favored during lateral decubitus, thus the ventilation to perfusion match results less affected, decreasing the risk of hypoxemia. It also reduces the risk of atelectasis in the non-operative lung by keeping diaphragm contraction. The use of propofol and other agents (mepivacaine, ropivacaine) in the thoracic epidural catheter seems to keep the physiological mechanism of HPV, which in conclusion favors perfusion on the non-operative ventilated lung. Avoiding orotracheal intubation and MV clearly diminishes the risk of barotrauma, volutrauma, atelectrauma and the inflammatory response to the aggression of manipulating the airway. Given that COPD patients show a high incidence of bronchial hyperreactivity, avoiding GA and tracheal intubation can prevent from bronchospasm, which in some situations can be lethal for these patients (34-37). Alongside this, the earlier mobilization and restart of oral intake decreases the risk of developing atelectasis and secretions retention, so all of this may explain the reduction in postoperative respiratory complications (20,23). On the other hand, some negative effects of iatrogenic pneumothorax in a spontaneously breathing patient are not negligible. Mediastinum drop above the non-operative lung due to the atmospheric pleural pressure and rebreathing mechanism of expirated air into the dependent lung can produce some degree of hypoxemia, and hypercapnia (38). Oxygen delivery to the patient can counteract these effects by increasing oxygen saturation in the inspirated air in order to elevate oxygen alveolar pressure. Hypercapnia has been called “permissive” because usually seems to be well tolerated by patients and seldom forces to convert to GA. All of those beneficial effects are especially important in patients with severely depressed pulmonary reserve and poor general status, because they might signify the difference between developing or not postoperative complications. NI-VATS experience with other kind of procedures aims at reduction in surgical time but mainly in OR time, thus potentially increasing the OR occupation ratio, and also aims at reducing hospital stay which could have an economical benefit reducing procedure-related costs (39). Finally, Pompeo et al. (40) reported no differences identifying emphysema-like changes (ELCs) during surgery for PSP, between the NI-VATS and the GA arms (90% vs. 95%; P=0.52), and these findings are in agreement with those reported by Nezu, suggesting that it can even facilitate detection of small blebs/bullae which remained insuflated during spontaneous breathing.
But, which are the results in terms of benefits and risks obtained by surgical groups performing non-intubated VATS procedures for patients with SSP? Table 1 resumes the main results to date. Best evidence collected proceeds from the only two comparative studies by Nezu et al. (29) and Noda et al. (23). Significant decrease in operative time was found by Nezu when comparing with the GA arm (44.6±11.6 vs. 63.3±20 minutes, P<0.01), but this difference could not be statistically proven when comparing the TEA/local arm vs. GA arm in the report by Noda in 2012, although they reported a trend for reducing this operative time in the TEA arm (85.9±35.3 vs. 111.4±56.1 minutes) not reaching the statistical significance (P=0.12). Significant difference in total OR time favoring the non-intubated arm (116.5±5 vs. 209.1±77.1 minutes) both in the entire comparison and in the propensity score-matched comparison (P<0.0001 and P=0.006) was reported in this latter study. Postoperative complications incidence favors the NI-VATS groups in both studies (26.6% vs. 30.9% in Noda, and 9.3% vs. 10.5% in Nezu) not reaching statistical significance, but in a subanalysis by Noda, they describe significant reduction of respiratory complications which benefits the TEA arm both in the total entire cohort study and in the propensity score-matched analysis (P=0.046 and P=0.02, respectively), even though the TEA patients had poorer PS as shown in Table 1. Finally Nezu found a non-negligible reduction in hospital stay in the NI-VATS arm (4.5±1.3 vs. 5.8±1.1 days, P<0.01) although not finding differences in the drainage time (3.3±0.9 vs. 3.7±1.3 days). A trend toward reducing hospital death in the more affected patients of the TEA arm was mentioned by Noda, despite not reaching significance (0 deaths against 4 deaths in the GA group). Recurrence rate was not increased in the local anesthesia group analyzed by Nezu (3.2%) when compared to the 2.8-9.3% rate in present studies of surgical result in SSP under GA (12,41,42). Globally it seems that NI-VATS can be performed in these patients safely and even decreasing postoperative complications, and it can also save surgical/OR time and hospital stay when comparing to the standard GA regimen. These results support the theoretical benefits previously mentioned and it also seems not to increase the risk for a future recurrence in severely affected patients.
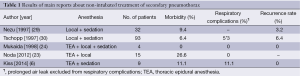
Full table
Case series by Mukaida (24) and Kiss (6) show the feasibility and safety of managing high risk patients with a NI-VATS approach without recurrences in the follow-up interval, and reasonable results in terms of postoperative complications with only one ADRS in the study by Kiss in a patient with severe interstitial pulmonary fibrosis and ASA 4 who died on the POD 25. These results constitute a real alternative for patients that normally would be rejected for standard procedures under GA because of high risk.
Some case reports published (25,26) also bring to mind that NI-VATS with thoracic epidural and/or local anesthesia has been anecdotally proven feasible and safe for managing patients with special basal situations that contraindicate or at least increase the risks for complications if GA and one-lung MV are administered, such as lung transplantation or contralateral pneumonectomy, thus providing a new choice with a future potential that must be elucidated.
Specific surgical management of SSP in patients with TEA and/or local anesthesia is not far different from the standard procedures and manoeuvres used for this surgery under GA, highlighting the coincidence in resecting the bullas when possible (Figure 5) but trying to seal the leak with fibrin glues/polyglycolic acid sheets in the cases of strong adhesions in order to avoid iatrogenic damage. But surprisingly, barely one of these reports (30) mentions a method of creating a pleural symphysis for preventing recurrences, such as abrasion pleurodesis, apical pleurectomy of chemical pleurodesis with talc, which are recommended in the American College Chest Physicians (5), Respiratory Pathology Spanish Society (3) and British Thoracic Society guidelines (1) of 2001, 2008 and 2010 respectively.
When dealing with potential future lines of research in this field, some ethical issues should be considered. There is evidence that the risk for ventilator dependency in patients with comorbidities is higher in cases of GA (43), and usually SSP patients have poorer PS and cardiopulmonary underlying disease. There is no comparative study of SSP patients undergoing surgery with TEA/local anesthesia against GA which mentions ASA status, but the report by Kiss proved the feasibility and safety of surgery under TEA in ASA 4 patients rejected for GA, in terms of morbidity and recurrence. Also the report by Noda included patients with poorer PS and oxygen requirement in the TEA group, with better results in terms of morbidity and respiratory complications when comparing to patients under GA. It remains unclear whether NI-VATS surgery under TEA/local anesthesia in ASA 3-4 patients could potentially benefit in terms of morbidity and mortality, so a randomized prospective clinical trial would be required to elucidate this dilemma. But as many of these patients are usually contraindicated for GA and because of the encouraging results under NI-VATS surgery, setting up a randomized clinical trial in a real hospital clinical setting results controversial at least.
There is some heterogeneity in the surgical treatment of SSP: some groups tend to treat only the air leak (mainly bullas) with different strategies, plicating the bulla, resection with an endostapler, or applying fibrin glue and polyglycolic acid sheets, in most cases. In the other hand some groups perform pleurodesis to produce pleural symphysis. Finally some groups treat the air leak but also perform pleurodesis with different mechanisms, although criteria for the latter usually lack and is left to the surgeon’s decision. This heterogeneity in the surgical management hinders the research, because it adds more variability, and it is also related to the broad period where these studies have been performed, since 1997 till 2014, when the directives in the treatment of SSP have slowly changed. We need homogeneous comparable surgical and anesthetic strategies when comparing TEA/local anesthesia with GA, in order to identify which aspects directly contribute to the results in terms of morbidity, mortality and recurrences. There is no literature of non-intubated uniportal VATS surgery in the treatment of pneumothorax, although our group has performed already three procedures for PSP safely, so more experience is needed to assess the potential benefits of uniportal approach in these patients.
Despite the difficulty, a multicenter international database could be created in order to collect the patients with SSP who finally undergo surgery in the centers performing NI-VATS, including both patients operated with TEA and/or local anesthesia or GA. Although limited for setting strong recommendations, this database may contribute to accumulate data about which patients benefit more of a NI-VATS procedure, risk factors for morbidity and mortality in both groups, and which anesthetic and surgical regimen could benefit more these patients.
Conclusions
NI-VATS with TEA and/or local anesthesia is a feasible and safe alternative for patients with persistent or recurrent SSP with underlying pulmonary disease. The results of the last two decades aim at reduction of surgical and OR times, hospital stay, and decrease in postoperative morbidity and respiratory complications, when compared to GA. It also constitutes an alternative for patients rejected for GA because of their high risk of ventilator dependency. Moreover there is a lack of prospective randomized clinical trials to achieve stronger evidence to make firm recommendations about the surgical treatment in these patients.
Acknowledgements
When the doctor becomes the patient, he experiences desolation, affliction and hopelessness. Then he needs the words to keep on fighting: endure, dream, live. To my extraordinary wife, for all. To my beloved family, for peacefulness. To my friends, for support. Thanks to life.
Authors’ contributions: C Galvez, MD: performed literature search, designed the review, wrote the paper and finally approved the version to be published; S Bolufer, MD: contributed to conception and design of the review, analyzed and revised the content of the paper, and proofread the language; J Navarro-Martinez, MD, DEAA: contributed to conception and design of the review, analyzed and revised the content of the paper, and proofread the language; F Lirio, MD: contributed to conception and design of the review, analyzed and revised the content of the paper; JM Corcoles, MD: contributed to conception and design of the review, analyzed and revised the content of the paper; JM Rodriguez-Paniagua, MD: contributed to conception and design of the review, analyzed and revised the content of the paper.
Disclosure: The authors declare no conflict of interest.
References
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Vanni G, Tacconi F, Mineo TC, et al. Awake thoracoscopic treatment of spontaneous pneumothorax. In: Pompeo E. eds. Awake Thoracic Surgery (Ebook). Sharja: U.A.E., Bentham Science Publishers, 2012:130-40.
- Rivas de Andrés JJ, Jiménez López MF, Molins López-Rodó L, et al. Guidelines for the diagnosis and treatment of spontaneous pneumothorax. Arch Bronconeumol 2008;44:437-48. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Kiss G, Claret A, Desbordes J, et al. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg 2014;19:816-23. [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Szegedi LL, Bardoczky GI, Engelman EE, et al. Airway pressure changes during one-lung ventilation. Anesth Analg 1997;84:1034-7. [PubMed]
- Tusman G, Böhm SH, Sipmann FS, et al. Lung recruitment improves the efficiency of ventilation and gas exchange during one-lung ventilation anesthesia. Anesth Analg 2004;98:1604-9. [PubMed]
- Williams EA, Quinlan GJ, Goldstraw P, et al. Postoperative lung injury and oxidative damage in patients undergoing pulmonary resection. Eur Respir J 1998;11:1028-34. [PubMed]
- Nakajima J. Surgery for secondary spontaneous pneumothorax. Curr Opin Pulm Med 2010;16:376-80. [PubMed]
- Zhang Y, Jiang G, Chen C, et al. Surgical management of secondary spontaneous pneumothorax in elderly patients with chronic obstructive pulmonary disease: retrospective study of 107 cases. Thorac Cardiovasc Surg 2009;57:347-52. [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Diaphragm motion in the operative lung during NI-VATS procedures. Asvide 2015;2:041. Available online: http://www.asvide.com/articles/496
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126-30. [PubMed]
- Nagendran J, Stewart K, Hoskinson M, et al. An anesthesiologist's guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anaesthesiol 2006;19:34-43. [PubMed]
- Pruszkowski O, Dalibon N, Moutafis M, et al. Effects of propofol vs sevoflurane on arterial oxygenation during one-lung ventilation. Br J Anaesth 2007;98:539-44. [PubMed]
- Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: a meta-analysis. Anesth Analg 2001;93:853-8. [PubMed]
- Wijeysundera DN, Beattie WS, Austin PC, et al. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet 2008;372:562-9. [PubMed]
- Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg 1998;86:598-612. [PubMed]
- Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000;321:1493. [PubMed]
- Pöpping DM, Elia N, Marret E, et al. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 2008;143:990-9; discussion 1000. [PubMed]
- Baird AG, Lawrence JR. Guidelines: is bigger better? A review of SIGN guidelines. BMJ Open 2014;4:e004278. [PubMed]
- Noda M, Okada Y, Maeda S, et al. Is there a benefit of awake thoracoscopic surgery in patients with secondary spontaneous pneumothorax? J Thorac Cardiovasc Surg 2012;143:613-6. [PubMed]
- Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg 1998;65:924-6. [PubMed]
- Sugimoto S, Date H, Sugimoto R, et al. Thoracoscopic operation with local and epidural anesthesia in the treatment of pneumothorax after lung transplantation. J Thorac Cardiovasc Surg 2005;130:1219-20. [PubMed]
- Noda M, Okada Y, Maeda S, et al. Successful thoracoscopic surgery for intractable pneumothorax after pneumonectomy under local and epidural anesthesia. J Thorac Cardiovasc Surg 2011;141:1545-7. [PubMed]
- Pompeo E; Awake Thoracic Surgery Research Group. To be awake, or not to be awake, that is the question. J Thorac Cardiovasc Surg 2012;144:281-2; author reply 282. [PubMed]
- Mineo TC, Ambrogi V. Awake thoracic surgery for secondary spontaneous pneumothorax: another advancement. J Thorac Cardiovasc Surg 2012;144:1533-4. [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230-5. [PubMed]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [PubMed]
- de la Torre Bravos M, Rivas de Andrés JJ. Treatment of pneumothorax with VATS and bullectomy under local anesthesia. Video assisted thoracic surgery. Ann Thorac Surg 1999;68:2383. [PubMed]
- Yutaka Y, Katakura H, Kaneda S, et al. Local anaesthetic thoracoscopy for intractable pneumothorax in a high-risk patient. Interact Cardiovasc Thorac Surg 2012;15:330-1. [PubMed]
- Pompeo E. Pathophysiology of surgical pneumothorax in the awake patient. In: Pompeo E. eds. Awake thoracic surgery (Ebook). Sharja: U.A.E., Bentham Science Publishers, 2012:9-18.
- Boushey HA, Holtzman MJ, Sheller JR, et al. Bronchial hyperreactivity. Am Rev Respir Dis 1980;121:389-413. [PubMed]
- Ramsdell JW, Nachtwey FJ, Moser KM. Bronchial hyperreactivity in chronic obstructive bronchitis. Am Rev Respir Dis 1982;126:829-32. [PubMed]
- Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: a closed claims analysis. Anesthesiology 1990;72:828-33. [PubMed]
- Warner DO, Warner MA, Offord KP, et al. Airway obstruction and perioperative complications in smokers undergoing abdominal surgery. Anesthesiology 1999;90:372-9. [PubMed]
- Benumof JL. Chapter 2: distribution of ventilation and perfusion. In: Benumof JL. eds. Anesthesia for thoracic surgery. 2nd edition. Philadelphia: WB Saunders, 1995:35-52.
- Pompeo E, Mineo TC. Awake operative videothoracoscopic pulmonary resections. Thorac Surg Clin 2008;18:311-20. [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [PubMed]
- Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg 2013;17:247-52. [PubMed]
- Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax--long-term results. Eur J Cardiothorac Surg 2011;40:120-3. [PubMed]
- de Albuquerque Medeiros R, Faresin S, Jardim J. Postoperative lung complications and mortality in patients with mild-to-moderate COPD undergoing elective general surgery. Arch Bronconeumol 2001;37:227-34. [PubMed]


