Pneumothorax as a complication of central venous catheter insertion
Introduction
Pneumothorax is the one of the most frequent mechanical complications during central venous catheter (CVC) insertion. CVC insertion is a commonly performed procedure which facilitates resuscitation, nutritional support, and long-term vascular access. The clinical use of CVC was first described by Aubaniac in 1952 as a method of resuscitating trauma patients on the battlefield (1).
There has been an increase in the use of CVCs over the last decade due to the increase in disease severity, age, and severe co-morbidity of patients, especially in the intensive care unit (ICU) setting. Moreover, medical treatment for various clinical conditions has become more intense and prolonged, with concomitant increases in morbidity and the need for supportive medical care. As a result of all these factors the incidence and severity of mechanical and other catheter-related complications have increased. Clinicians now insert millions of CVCs annually, with an overall complication rate of 15%, ranging from 5% to 19% (2-5). Serious complications may significantly add to morbidity and mortality of already compromised patients and also lead to a considerable increase in the cost of treatment.
The complications of central vein catheterization include infection, thrombosis, occlusion, and, in particular, mechanical complications which usually occur during insertion and are closely related to the anatomic location of the central veins. Infectious complications are reported to occur in 5% to 26% of patients, mechanical complications in 5% to 19%, and thrombotic complications in 2% to 26% (4,5). Mechanical complications associated with the insertion of central lines include arterial puncture, hematoma, hemothorax, pneumothorax, arterial-venous fistula, venous air embolism, nerve injury, thoracic duct injury (left side only), intraluminal dissection, and puncture of the aorta (6).
Pneumothorax is one of the most common CVC insertion complications, reportedly representing up to 30% of all mechanical adverse events of CVC insertion (7). The incidence of pneumothorax varies between 1% and 6.6%, with higher incidences being reported in the following situations: emergency situations, large catheters size, catheters used for dialysis, and with the increased number of needle passes (8). Subclaian vein insertion (SCV) has been reported to have a higher incidence of pneumothorax than internal jugular vein (IJV) insertion (4,9).
The likelihood of mechanical complications is largely determined by three categories of factors:
- Patient-related factors:
- Nature of the underlying disease and the presence of comorbidity (e.g., pulmonary emphysema/COPD, coagulopathy);
- Anatomy of the patient, (abnormal body mass index, abnormal weight-to-height ratio), congenital anomalies such as persistent left superior vena cava;
- Compromised procedural settings (mechanical ventilation or emergency);
- Patients restlessness or uncooperative patients;
- Previous operations, trauma or radiotherapy in the anatomic region of interest.
- Catheter-related factors:
- Site chosen for CVC insertion;
- Catheter type.
- Clinical factors:
- Experience of the physician inserting the CVC;
- Previous catheterizations;
- Catheterization attempts;
- Emergency or elective situations.
The level of experience of the physician inserting the CVC is of paramount importance. Insertion of a catheter by a physician who has performed 50 or more catheterizations is half as likely to result in a mechanical complication as insertion by a physician who has performed fewer than 50 catheterizations (10). Previous unsuccessful insertion attempts, especially in an emergency setting are the most common predictors of insertion complications (8). The number of needle passes was strongly associated with the rates of failure and complications. It has been reported that with one attempt of catheterization the complication rate is 4.3% which rises to 24% in patients who undergo more than two attempts (11).
In a prospective study of Schummer et al. the incidence of primary mechanical complications and malpositions associated with landmark-guided central venous access procedures by experienced operators were analyzed in the ICU setting (12). The incidence of pneumothorax was not related to the insertion site. More than one cannulation attempt was associated with a significantly higher mechanical complication rate. The risk of mechanical complications, including failure, increased nearly 10 times with two attempts and the majority of mechanical complications occurred in procedures involving more than two attempts. The authors concluded that even experienced operators cause a considerable number of early mechanical complications and malpositions, after two unsuccessful cannulation attempts failure and associated complications are very likely.
Kilbourne et al. (13) reported that if only one needle pass was attempted during subclavian CVC placement, the failure rate for subsequent catheter placement was 1.6%, compared with 10.2% for two attempts and 43.2% for three or more attempts. Risk of mechanical complications is dramatically increased if the local anatomy is altered (cannulation of the jugular or subclavian vein after sternotomy or clavicular fracture) (14). In this regard, it is noteworthy that connections between the pleural cavities can develop after thoracic surgery. Thus, CVC insertion on one side can lead to contra-lateral or bilateral complications, especially in patients with a history of thoracic surgery. Similarly, the risk may be increased in patients with the narrow thoracic inlet syndrome.
Large catheter size, such as those used for dialysis, appears to increase the risk of vascular complications of insertion (6,8).
Apart from patient and procedural factors, most CVC-related complications are closely linked to the insertion site, usually the jugular, subclavian or femoral vein.
Sites of central vascular access and related risk of complications
Overall, internal jugular catheterization and subclavian venous catheterization carry similar risks of mechanical complications. Subclavian catheterization is more likely than internal jugular catheterization to be complicated by pneumothorax and hemothorax, whereas internal jugular catheterization is more likely to be associated with arterial puncture. Hematoma and arterial puncture are common during femoral venous catheterization (4).
Studies in normal risk patients found a higher incidence of pneumothorax when the subclavian vein is cannulated, as compared with the IJV (0.5-2% vs. 0.2-0.5%) (9,15,16). Subclavian venous catheterization has occasionally been linked to a lower incidence of pneumothorax than IJV access (17). The frequency of mechanical complications according to the sites of central vascular access is presented in Table 1.
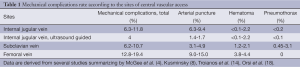
Full table
Internal jugular catheterization can be difficult in obese patients, in whom the landmarks of the neck are often obscured. Subclavian venous catheterization should be avoided in patients with severe hypoxemia, because the complication of pneumothorax is more likely to occur at this site and is less likely to be tolerated by such patients. When inserting a CVC in the jugular or subclavian veins of patients with unilateral pulmonary disorders (pneumonia, traumatic pneumothorax, etc.), this should, if possible, be performed on the side of the affected lung as the consequences of potential complications will be less severe.
If central venous access is needed for resuscitation from shock, femoral venous access should be considered because of the speed with which it can be performed, especially if it is believed that internal jugular or subclavian venous catheterization will be difficult. Conversely, a prospective, comparative study suggests that during cardiac arrest the catheterization success rate can be higher for SCV than for FV access (19).
Recognition of pneumothorax: symptoms and signs
Until recently it was common practice to exclude radiologically detectable complications after the insertion of CVCs by the jugular or subclavian approach by chest X-ray. However, this procedure is expensive, carries the risk of cancer induction, may delay more important procedures and may even lead to a false sense of safety because not all complications are visible on X-rays shortly after the procedure and not all visible complications are correctly diagnosed (20-22).
Molgaard et al. (16) found no value in routine X-ray control and considered the omission of routine chest X-ray control after CVC placement. In a prospective study Rowan et al. (23) compared the accuracy of ultrasonography (US) with that of supine chest radiography (CR) in the detection of traumatic pneumothoraces, with computed tomography (CT) as the reference standard. Supine chest radiographs were unreliable in making the diagnosis of pneumothorax, with a sensitivity value of 36%. US was more sensitive than supine CR and as sensitive as CT in the detection of pneumothoraces. Alrajhi et al. (24) in a systematic review and meta-analysis study, compared the test characteristics of US and supine CR in adult patients clinically suspected of having a pneumothorax, using CT scan findings or the release of air from the chest tube as a reference standard. Performance of US for the detection of pneumothorax is excellent and is superior to supine CR. CR data were available for 864 of 1,048 patients evaluated with US. US was 90.9% sensitive (95% CI, 86.5-93.9) and 98.2% specific (95% CI, 97.0-99.0) for the detection of pneumothorax. CR had 50.2% sensitivity (95% CI, 43.5-57.0) and 99.4% specificity (95% CI, 98.3-99.8). Considering the ease of access and the excellent clinical performance of US the study supports the routine use of US for the detection of pneumothorax.
CR of supine patients is not sensitive enough to identify hidden pneumothorax because the air initially dissipates within the nondependent and medial parts of the chest and therefore can be invisible on supine radiographs. Even upright CR can be challenging, however, because lines, tubes, and other folds can hide subtle pleural line abnormalities. Only when the air volume increases and pneumothorax extend to the apical and lateral sites where the separation of pleural layers is more easily identifiable does radiography have diagnostic value. CR is often performed immediately after CVC insertion and it is therefore possible that there is not enough time for the development of a pneumothorax large enough to be identified.
Although chest CT is quite accurate, it involves moving potentially unstable patients to a less monitored environment; it involves radiation exposure; and its increased cost makes it an inefficient screening tool.
Clinician-performed bedside US allows the diagnosis of pneumothorax to be made immediately, with a high degree of sensitivity and with better accuracy than supine chest films and equal to that of CT scan (14,23,25). Lung US scans carried out in the emergency department detect occult pneumothorax and its extension with an accuracy that is almost as high as the reference standard (CT scanning) (26). Sonography is portable, can be performed at the bedside, and has no risk associated with repeated measurements as clinical scenarios change. These advantages can make it less expensive because there are no additional burdens placed on radiologic technologists, and the performance is physician dependent. Indeed, numerous studies have described near 100% sensitivity and 90% to 95% specificity if a thorough examination is performed (27-29). The meta-analysis of Ding et al. (30) indicated that bedside US performed by clinicians had higher sensitivity and similar specificity in comparison to anterior-posterior CR in the diagnosis of pneumothorax, but the accuracy of US in the diagnosis of pneumothorax depended on the skill of the operators.
Given the ease of performance, the low cost, and the multiple clinical scenarios in which this diagnosis is considered, application of this examination could be considered in any situation in which a chest radiograph is being ordered to evaluate for pneumothorax. Given the frequency with which this diagnosis is considered in the hospital setting, using thoracic sonography as a screening tool may lead to decreased number of chest radiographs, may optimize recourse utilization, reduce costs and minimize unnecessary repeated radiation exposure of both patients and physicians (31-33).
Interventions to prevent and manage pneumothorax during CVC insertion
The profound impact of the complications associated with CVC use is so important that efforts to minimize and prevent its occurrence should be a routine element of quality improvement programs. The interventions to prevent and manage pneumothorax during CVC insertion are presented in Table 2.
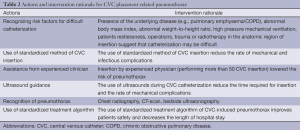
Full table
Treatment of pneumothorax after CVC insertion
If a pneumothorax is diagnosed the treatment strategy should be determined by the following factors: (I) size; (II) symptoms; (III) spontaneous breathing or use of mechanical ventilation; (IV) clinical diagnosis of a tension pneumothorax.
A standardized treatment algorithm of CVC-induced pneumothorax can safely lead to improvements in patients’ comfort, and decreases in the length of stay for both adults and children (8). Laronga et al. developed a treatment algorithm for iatrogenic pneumothorax complicating CVC insertion, based on the retrospective review of 100 pneumothorax cases secondary to subclavian CVC insertion on an outpatient basis (34).
Treatment consisted of: (I) observation; (II) outpatient insertion of a Heimlich valve; (III) inpatient tube thoracostomy. A total of 58 patients underwent observation as their initial treatment, and this strategy was successful in 35 patients (60%). A total of 34 patients were initially treated with the Heimlich valve, and this strategy was successful in 29 patients (85%). Tube thoracostomy as the initial therapy was successful in 7 (88%) of 8 patients. Patients with the Heimlich valve required a mean of 1.7 days of outpatient treatment as compared to 3.7 days of hospitalization for patients treated with tube thoracostomy.
On the basis of the results of the study, the authors proposed a treatment algorithm for pneumothoraces complicating CVC placement.
For pneumothoraces 30% or smaller, in asymptomatic patients, in whom there was no difficulty with CVC insertion, a trial of observation is appropriate. Inspiration/expiration CR performed four hours later, followed by a CR performed 24 hours later should be obtained to assess the stability of pneumothorax. If the pneumothorax enlarges and the patient develops symptoms a small pigtail catheter with a Heimlich valve can be inserted on an outpatient basis. For larger pneumothoraces (>30%), for symptomatic patients, and for patients with a prior history of same-side CVC insertion a pigtail catheter can be used as the initial treatment. Large—tube thoracostomy can be reserved for refractory pneumothoraces and in the emergency setting. However in critically ill patients under mechanical ventilation a small pneumothorax may quickly develop into a tension pneumothorax with a rapid deterioration of patient’s condition. A large thoracostomy tube is recommended as the initial treatment in mechanically ventilated patients with pneumothorax after CVC placement.
Standardized method of CVC insertion
Because the complication rate decreases with training, designing a standardized method of CVC insertion is a logical process to promote prevention and decrease the incidence of adverse events (4).
Ultrasonography-assisted insertion
The use of ultrasound guidance has been referred to as a method for reducing the risk of mechanical complications during insertion of CVC. Imaging of the target vein by ultrasound prior to catheter insertion is clinically useful to confirm the presence of a target vein of adequate size for cannulation, because nearly 10% of patients have abnormal venous anatomy, including the absence of the vein of interest (4,35). There is a broad consensus and an extensive body of evidence-based literature demonstrating that real-time ultrasound-guided CVC placement is associated with fewer overall and specific complications, improved catheter insertion success and reduced costs (6,14,36-39). Reports of the advantages of US guided insertion method over the anatomic landmark method support the findings of risk reduction and improved insertion success for all access sites and in different settings (37,40). In addition, the gap between experienced and inexperienced operators has been reported to disappear when UAI is used (41). Conversely, US-assisted insertion can be of help to a skillful operator who is otherwise unable to perform the CVC insertion (35).
Internal jugular vein (IJV)
The IJV is probably the most common site for CVL placement. The IJV is classically described as exiting the external jugular foramen at the base of the skull posterior to the internal carotid and coursing toward an anterolateral position (in relation to the carotid) as it travels caudally. Vascular anomalies and anatomic variations of this vessel and surrounding tissues have been observed in up to 36% of patients (42). Denys and Uretsky (35) showed that the IJV was located outside of the path predicted by landmarks in 5.5% of patients.
Ultrasound identifies the vein size and location, anomalies, and vessel patency, thus avoiding futile attempts in patients with absent or thrombosed veins and congenital anomalies such as persistent left superior vena cava. Troianos et al. (43) demonstrated that the overall success rate of central venous cannulation could be improved from 96% to 100% with the use of ultrasound. The improved first attempt success rate (from 54% to 73%), the decreased needle advances (from 2.8 to 1.4 attempts), the decreased time to CVC insertion (from 117 to 61 sec), and the lower rate of arterial punctures (from 8.43% to 1.39%) have been demonstrated in this study. Obese patients and patients with obscured external landmarks derive a particular benefit from ultrasound guidance by decreasing the incidence of arterial puncture, hematoma formation, and pneumothorax (44,45).
The recognition and avoidance of pleural tissue during real-time ultrasound imaging could potentially decrease the risk for pneumothorax for approaches that involve a needle entry site closer to the clavicle (14).
Oguzkurt et al. (46) prospectively reviewed the placement of 220 temporary IJV dialysis catheters using US guidance in a high risk, patients with 100% success rate, with only seven mechanical complications among the 171 procedures.
In summary, ultrasound guidance reduces the number of mechanical complications, the number of catheter placement failures, and the time required for IJV insertion.
Subclavian vein
Landmark-guided SC vein access uses the anatomic landmarks of the midpoint of the clavicle, the junction between the middle and medial border of the clavicle, and the lateral aspect of a tubercle palpable on the medial part of the clavicle. Advantages of using the subclavian vein for central venous access include consistent surface anatomic landmarks and vein location, patient comfort, and lower potential for infection (14).
Complication rates for the landmark-guided approach to SC vein cannulation are 0.3% to 12% and include pneumothorax, hematoma, arterial puncture, hemothorax, catheter malposition, and thoracic duct laceration (left side only) (11). Kilbourne et al. (13) reported the one of the most common errors during failed SC vein catheter placement attempts by resident physicians was inadequate landmark identification of the vessel. Higher success rate in patients with ultrasound guidance as compared to those with landmark-guided approach was demonstrated in a study of Orsi et al. (18). The ultrasound group required fewer attempts, showed better patient compliance, and a zero incidence of pneumothorax, while the incidence of pneumothorax in the landmark group was 4.8%. A prospective randomized SC vein cannulation study performed by Gualtieri et al. (47) favored the ultrasound-guided over the landmark-guided approach, with a higher success rate (92% vs. 44%), fewer minor complications (1 vs. 11), and fewer venopunctures (1.4 vs. 2.5) and catheter kits (1.0 vs. 1.4) per attempted cannulation.
However, the use of ultrasound guidance during subclavian venous catheterization has had mixed results in clinical trials, the fixed anatomical relation between the subclavian vein and the clavicle makes ultrasound-guided catheter insertion more difficult and less reliable than landmark-based insertion (14).
Recommendations for central vein placement using ultrasound guidance
Members of American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists, the panel of experts in ultrasound-guided catheter insertion developed consensus definitions on ultrasound cannulation (14).
Recommendations of American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists
Recommendation for subclavian vein cannulation
Current literature does not support the routine use of ultrasound for uncomplicated patients undergoing SC vein cannulation. Individual operators should not attempt cannulation more than twice, as the incidence of complication, particularly pneumothorax, rises significantly with additional attempts. High-risk patients may benefit from ultrasound screening of the SC vein before attempted cannulation to identify vessel location and patency and to specifically identify thrombus before attempted cannulation. The recommendation for ultrasound guidance during SC vein cannulation is based on category A (supportive), level 3 evidence.
Recommendation for IJV cannulation
It is recommended that properly trained clinicians use real-time ultrasound during IJ cannulation whenever possible to improve cannulation success and reduce the incidence of complications associated with the insertion of large-bore catheters. This recommendation is based on category A, level 1 evidence.
International evidence-based recommendations on ultrasound-guided vascular access
International experts in ultrasound-guided cannulation analyzed the randomised clinical trials performed during the last decade and concluded that there are growing clinical experience showing that the benefits of ultrasound-guided venipuncture can be extended to all venous access sites (48).
Recommendations: (I) ultrasound guidance should be routinely used for short-term central venous access in adults. Level of evidence-A; degree of consensus-very good; strength of recommendation-strong; (II) ultrasound guidance should be routinely used for long-term central venous access in adults. Level of evidence-A; degree of consensus-very good; strength of recommendation-strong; (III) ultrasound can accurately detect pneumothorax and should be routinely performed after CVC cannulation when the pleura could have been damaged. Level of evidence-b; degree of consensus-very good; strength of recommendation-strong.
Limitations of US guided CVC insertion method
The US guided CVC insertion approach has not yet gained widespread acceptance, it is operator-dependent, and patient selection and equipment can influence the results (49-64).
The three main limitations for US application in regard to vascular access are: (I) US physics and their incompatibility; (II) learning curve; (III) the need for special equipment and its price (65-79).
As with all new techniques, ultrasound-guided catheterization requires training. In hospitals where ultrasound equipment is available and physicians have adequate training, the use of ultrasound guidance should be routinely considered for central venous catheterization (78,80-94).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Aubaniac R. Subclavian intravenous injection; advantages and technic. Presse Med 1952;60:1456. [PubMed]
- Veenstra DL, Saint S, Saha S, et al. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 1999;281:261-7. [PubMed]
- Sznajder JI, Zveibil FR, Bitterman H, et al. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med 1986;146:259-61. [PubMed]
- McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123-33. [PubMed]
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286:700-7. [PubMed]
- Polderman KH, Girbes AJ. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med 2002;28:1-17. [PubMed]
- Mitchell SE, Clark RA. Complications of central venous catheterization. AJR Am J Roentgenol 1979;133:467-76. [PubMed]
- Kusminsky RE. Complications of central venous catheterization. J Am Coll Surg 2007;204:681-96. [PubMed]
- Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. J Intensive Care Med 2006;21:40-6. [PubMed]
- Bernard RW, Stahl WM. Subclavian vein catheterizations: a prospective study. I. Non-infectious complications. Ann Surg 1971;173:184-90. [PubMed]
- Mansfield PF, Hohn DC, Fornage BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med 1994;331:1735-8. [PubMed]
- Schummer W, Schummer C, Rose N, et al. Mechanical complications and malpositions of central venous cannulations by experienced operators. A prospective study of 1794 catheterizations in critically ill patients. Intensive Care Med 2007;33:1055-9. [PubMed]
- Kilbourne MJ, Bochicchio GV, Scalea T, et al. Avoiding common technical errors in subclavian central venous catheter placement. J Am Coll Surg 2009;208:104-9. [PubMed]
- Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011;24:1291-318. [PubMed]
- Kaiser CW, Koornick AR, Smith N, et al. Choice of route for central venous cannulation: subclavian or internal jugular vein? A prospective randomized study. J Surg Oncol 1981;17:345-54. [PubMed]
- Molgaard O, Nielsen MS, Handberg BB, et al. Routine X-ray control of upper central venous lines: Is it necessary? Acta Anaesthesiol Scand 2004;48:685-9. [PubMed]
- Miller JA, Singireddy S, Maldjian P, et al. A reevaluation of the radiographically detectable complications of percutaneous venous access lines inserted by four subcutaneous approaches. Am Surg 1999;65:125-30. [PubMed]
- Orsi F, Grasso RF, Arnaldi P, et al. Ultrasound guided versus direct vein puncture in central venous port placement. J Vasc Access 2000;1:73-7. [PubMed]
- Emerman CL, Bellon EM, Lukens TW, et al. A prospective study of femoral versus subclavian vein catheterization during cardiac arrest. Ann Emerg Med 1990;19:26-30. [PubMed]
- Farrell J, Walshe J, Gellens M, et al. Complications associated with insertion of jugular venous catheters for hemodialysis: the value of postprocedural radiograph. Am J Kidney Dis 1997;30:690-2. [PubMed]
- Bailey SH, Shapiro SB, Mone MC, et al. Is immediate chest radiograph necessary after central venous catheter placement in a surgical intensive care unit? Am J Surg 2000;180:517-21; discussion 521-2. [PubMed]
- Kaufman B, Dahr P, O'Niell DK, et al. Chest radiograph interpretation skills of anesthesiologists. J Cardiothorac Vasc Anesth 2001;15:680-3. [PubMed]
- Rowan KR, Kirkpatrick AW, Liu D, et al. Traumatic pneumothorax detection with thoracic US: correlation with chest radiography and CT--initial experience. Radiology 2002;225:210-4. [PubMed]
- Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012;141:703-8. [PubMed]
- Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med 2005;12:844-9. [PubMed]
- Soldati G, Testa A, Sher S, et al. Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008;133:204-11. [PubMed]
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010;17:11-7. [PubMed]
- Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999;25:383-8. [PubMed]
- Dulchavsky SA, Schwarz KL, Kirkpatrick AW, et al. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma 2001;50:201-5. [PubMed]
- Ding W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011;140:859-66. [PubMed]
- Peris A, Tutino L, Zagli G, et al. The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg 2010;111:687-92. [PubMed]
- Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med 2010;38:533-8. [PubMed]
- Maury E, Guglielminotti J, Alzieu M, et al. Ultrasonic examination: an alternative to chest radiography after central venous catheter insertion? Am J Respir Crit Care Med 2001;164:403-5. [PubMed]
- Laronga C, Meric F, Truong MT, et al. A treatment algorithm for pneumothoraces complicating central venous catheter insertion. Am J Surg 2000;180:523-6; discussion 526-7. [PubMed]
- Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med 1991;19:1516-9. [PubMed]
- Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996;24:2053-8. [PubMed]
- Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003;327:361. [PubMed]
- Abboud PA, Kendall JL. Ultrasound guidance for vascular access. Emerg Med Clin North Am 2004;22:749-73. [PubMed]
- Gann M, Sardi A. Improved results using ultrasound guidance for central venous access. Am Surg 2003;69:1104-7. [PubMed]
- Miller AH, Roth BA, Mills TJ, et al. Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department. Acad Emerg Med 2002;9:800-5. [PubMed]
- Geddes CC, Walbaum D, Fox JG, et al. Insertion of internal jugular temporary hemodialysis cannulae by direct ultrasound guidance—a prospective comparison of experienced and inexperienced operators. Clin Nephrol 1998;50:320-5. [PubMed]
- Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med 2001;22:23-6. [PubMed]
- Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg 1991;72:823-6. [PubMed]
- Beaulieu Y, Marik PE. Bedside ultrasonography in the ICU: Part 2. Chest 2005;128:1766-81. [PubMed]
- Giacomini M, Iapichino G, Armani S, et al. How to avoid and manage a pneumothorax. J Vasc Access 2006;7:7-14. [PubMed]
- Oguzkurt L, Tercan F, Kara G, et al. US-guided placement of temporary internal jugular vein catheters: immediate technical success and complications in normal and high-risk patients. Eur J Radiol 2005;55:125-9. [PubMed]
- Gualtieri E, Deppe SA, Sipperly ME, et al. Subclavian venous catheterization: greater success for less experienced operators using ultrasound guidance. Crit Care Med 1995;23:692-7. [PubMed]
- Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 2012;38:1105-17. [PubMed]
- Martin MJ, Husain FA, Piesman M, et al. Is routine ultrasound guidance for central line placement beneficial? A prospective analysis. Curr Surg 2004;61:71-4. [PubMed]
- Pirotte T. Ultrasound-guided vascular access in adults and children: beyond the internal jugular vein puncture. Acta Anaesthesiol Belg 2008;59:157-66. [PubMed]
- Kioumis IP, Zarogoulidis K, Huang H, et al. Pneumothorax in cystic fibrosis. J Thorac Dis 2014;6:S480-7. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. J Thorac Dis 2014;6:S470-9. [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Penetrating trauma. J Thorac Dis 2014;6:S461-5. [PubMed]
- Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis 2014;6:S448-60. [PubMed]
- Huang Y, Huang H, Li Q, et al. Transbronchial lung biopsy and pneumothorax. J Thorac Dis 2014;6:S443-7. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Li Z, Huang H, Li Q, et al. Pneumothorax: observation. J Thorac Dis 2014;6:S421-6. [PubMed]
- Huang Y, Huang H, Li Q, et al. Approach of the treatment for pneumothorax. J Thorac Dis 2014;6:S416-20. [PubMed]
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis 2014;6:S407-15. [PubMed]
- Machairiotis N, Kougioumtzi I, Dryllis G, et al. Laparoscopy induced pneumothorax. J Thorac Dis 2014;6:S404-6. [PubMed]
- Ouellette DR, Parrish S, Browning RF, et al. Unusual causes of pneumothorax. J Thorac Dis 2014;6:S392-403. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis 2014;6:S377-82. [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6:S7-S20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD assessment test: a simple tool to evaluate disease severity and response to treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]


