
Analysis of the immunoglobin E molecular sensitization profile in children with allergic asthma and predictive factors for the efficacy of allergy immunotherapy
Introduction
Asthma, a heterogeneous disease characterized by a chronic inflammatory pulmonary process, affects 1–18% of the population in different countries (1) and is a major chronic noncommunicable disease addressed by the World Health Organization in the Thirteenth General Programme of Work 2019–2023 (2). It has recently been reported that asthma affects 45.7 million adults in China (3). Meanwhile, the prevalence of childhood asthma in China is also increasing rapidly, with the 2000 and 2010 national statistics showing the cumulative prevalence in children under 14 years of age to be 1.97% (4), and 3.02%, respectively (5). Allergens, especially inhaled types, are detrimental for asthmatic children. The house dust mite (HDM) is one of the most common inhaled allergens worldwide, with Dermatophagoides pteronyssinus (Der P) and Dermatophagoides farina (Der F) being the major strains. Previously reported research established that HDMs are the major aeroallergens among allergic rhinitis (AR) patients in central China, with a sensitization rate of over 90% (6). Although the incidence of allergic asthma in children increases, the immunoglobin E (IgE) molecular sensitization profile of allergic asthma remains underreported.
The treatment of asthma mainly includes inhaled glucocorticoids, β2-receptor agonists, and leukotriene receptor antagonist. Current therapies can effectively control symptoms but do not affect the underlying, dysregulated immune response. In recent years, some new and targeted therapies have emerged, such as anti–immunoglobin E (IgE) antibody (omalizumab) and anti-interleukin 5 (IL-5) antibody (7). Although omalizumab is safe and effective, it is expensive, and there are no current guidelines for discontinuation (8). Allergen immunotherapy (AIT) is an alternative therapy in patients with allergic asthma, and there is evidence that the application of AIT in this population can achieve substantial reductions in short-term symptoms and medication scores (9). AIT currently represents the only approach capable of modifying the immune system to provide beneficial effects that persist for years after treatment is discontinued (10). A randomized clinical trial found that a HDM sublingual AIT tablet was effective in adults with allergic asthma (11). AIT has a unique immunological rationale, since this approach is tailored to the specific IgE spectrum of an individual and modifies the natural course of the disease. In this respect, AIT has to be presently considered a prototype within precision medicine (12). Unfortunately, however, AIT does not work for everyone, and has a reported efficacy rate of 50.7–71%, with a considerable number of patients failing to respond (13-15). In addition, AIT requires 3–5 years to complete the course of administration, which can be intolerably long for the patients, and thus selecting suitable patients (which will likely to comply) is critical to the effective application of this approach. Previous studies have shown that the level of total IgE (tIgE) and IgE-specific activity (sIgE/tIgE) can predict the efficacy of AIT, such that, lower the tIgE, the larger the sIgE/tIgE, the better the efficacy (13,14).
The aim of this study was thus to investigate the IgE molecular sensitization profile of allergic asthma in children in order to provide guidance for the prediction of AIT efficacy. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-7314).
Methods
Patients
The study was conducted from August 2018 till March 2019 in the Respiratory Department of the Children’s Hospital of Chongqing Medical University. The study was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University (file no. 2018-02), and each participant’s statutory guardian signed the informed consent. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Patients enrolled in our study were those who, (I) were diagnosed with allergic asthma based on the recommendations of the Global Initiative for Asthma guidelines (http://ginasthma.org/), (II) showed positive skin prick tests (SPTs) to Der p or Der f of “+++” or above, (III) and were aged 6–18. The exclusion criteria were those with (I) with heart, lung, liver, kidney, or other organic diseases; (II) with bronchial foreign bodies, tuberculosis, or other lung diseases; and (III) those who had previously received AIT. All patients were evaluated by a physician face-to-face with a detailed questionnaire to establish demographic characteristics, a family history of allergic diseases, other history of allergic disease, cigarette exposure, mode of delivery, and body mass index (BMI).
Total IgE, allergen sIgE, and component sIgE tests
Total IgE (kU/L), allergen serum sIgE (kUA/L) and component sIgE were measured with the ImmunoCAP assay (ImmunoCAP-100®, Thermo Fisher Scientific, Sweden), following the manufacturer’s instructions. The sIgE tests included Der p, Der f, Blomia tropicalis (Blo t), cat dander, dog dander, egg white, milk, cockroach, shrimp, and crab, along with the Der p allergen components, Der p1 and Der p2. A sIgE level over 0.35 kUA/L was defined as positive. Positive IgE tests were categorized into 6 classes: class 1 (≥0.35 to <0.70 kUA/L), class 2 (≥0.70 to <3.50 kUA/L), class 3 (≥3.50 to <17.50 kUA/L), class 4 (≥17.50 to <50.00 kUA/L), class 5 (≥50.00 to <100.00 kUA/L), and class 6 (≥100.00 kUA/L).
Statistical analysis
All the data were analyzed by Graphpad Prism 7. Descriptive parameters, including means and standard deviations for normally distributed continuous data, frequencies, and percentages for categorical data, were calculated. Pearson’s χ2 test or Fisher’s exact test was used to determine the association between categorical variables. The Mann-Whitney U test was used to compare numerical data between groups, while Spearman’s rank test was used to assess correlations.
Results
Demographic characteristics of patients
As shown in Table 1, a total of 142 asthmatic children with positive SPT to Der p or Der f were included, including 93 males and 49 females with an average age of 8.49 years (range, 6–17 years). The patients enrolled in our study had an average course of disease of 3.70 years (range, 1–12 years), and 83 (58.45%) patients had a family history of allergy. Of those, 132 patients (92.95%) were diagnosed with AR, and 71 (50.00%) patients said they were being exposed to cigarettes in their home or living environment. More than half of the child patients (71.13%) were delivered by cesarean section.
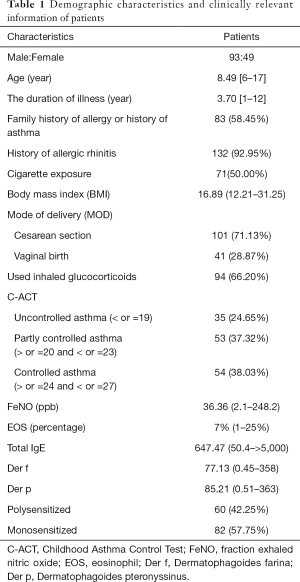
Full table
Prevalence of Serum IgE in HDM-allergic child asthma patients
Among the 142 allergic asthma patients, all of them (100%) showed positive IgE for Der p, Der p1, Der p2, and Der f, while the positive rates of Blo t, cat dander, dog dander, egg white, milk, cockroach, shrimp, and crab were 91.84%, 10.96%, 7.32%, 9.15%, 11.58, 17.03, 18.90, and 18.28% respectively (Figure 1A). Moreover, we found that the sIgEs of dust mites were higher than those of other allergens. The means of sIgE against Der p, Der p 1, Der p 2, and Der f were 92.59 (kUA/L), 80.50 (kUA/L), 82.62 (kUA/L), and 78.79 (kUA/L) respectively. While the means of sIgE against Blot, cat dander, dog dander, egg white, milk, cockroach, shrimp, and crab were 7.95 (kUA/L), 4.68 (kUA/L), 3.86 (kUA /L), 2.37 (kUA/L), 0.92 (kUA/L), 2.62 (kUA/L), 5.62 (kUA/L), and 5.43 (kUA/L), respectively (Figure 1B). Dust mites appear to be the major allergen among asthmatic children.
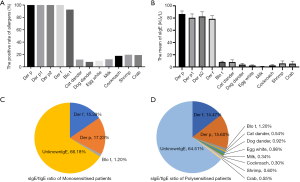
In this study, total IgE was divided into specific IgE and unknown IgE, which contains undetectable specific IgE and nonspecific IgE. Meanwhile, the patients were also divided into monosensitized patients, who are allergic only to dust mites, and polysensitized patients, who possessed more than one specific IgE sensitization against non-related allergen sources (16). In the monosensitized patient group, ratios of sIgE to Der p, Der f, and blot, relative to the total IgE were 17.23%, 15.39%, and 1.2% respectively, while the ratios of unknown IgE relative to the total IgE was 66.18% (Figure 1C). In the polysensitized patient group, ratios of sIgE of Der p, Der f, Blot, cat dander, dog dander, egg white, milk, cockroach, shrimp, and crab relative to total IgE were 15.60%, 14.47%, 1.20%, 0.54%, 0.92%, 0.98%, 0.34%, 0.30%, 0.60%, and 0.55% respectively, while the ratio of unknown IgE relative to total IgE was 64.51% (Figure 1D). There was no statistical difference between the monosensitized patient group and the polysensitized patients’ group in ratios of unknown IgE relative to total IgE (P>0.05).
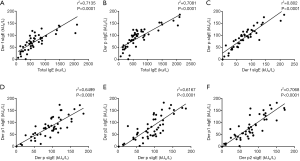
Correlations between total IgE and sIgE of Der f, Der p, Der p 1, and Der p 2
A significantly high correlation was found between total IgE and Der f sIgE (r2=0.7135, P<0.0001, Figure 2A), and a similar correlation was also found between total IgE and Der p sIgE (r2=0.7081, P<0.0001, Figure 2B). There was a high correlation between Der f sIgE and Der p sIgE (r2=0.802, P<0.0001, Figure 2C).Both Der p1 sIgE and Der p2 sIgE levels were highly correlated with Der p sIgE (r2=0.6499 and 0.6167, P<0.0001, respectively; Figure 2D,E). A high correlation between Der p1 sIgE and Der p2 sIgE (r2=0.7068, P<0.0001, Figure 2F). However, there was no significant correlation between Blo t and total IgE, Der p, Der f, Der p1, and Der p2.
The levels of total IgE, unknown IgE, and sIgE, and the sIgE/tIgE ratio in patients with and without a family history of allergy
Based on the presence of family history of allergy, all the allergic asthma patients were categorized into two groups. One group consisted of patients with a positive family history of allergy and the other group consisted of patients with no family history of allergy. We found that both total IgE and unknown IgE were higher in the positive family history of allergy group than in the negative family history of allergy group (Figure 3A). However, Der f and Der p sIgE levels showed no difference between these two groups (Figure 3B). Interestingly, the ratios of Der f sIgE/tIgE and Der p sIgE/tIgE were higher in the negative family history of allergy group than in the positive family history of allergy group (Figure 3C). The results suggested that a family history of allergy may affect the level of total IgE and the ratio of sIgE/tIgE.
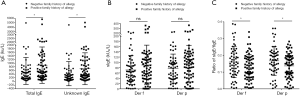
The levels of total IgE, unknown IgE, and sIgE, and the sIgE/tIgE ratio in monosensitized patients and polysensitized patients
Finally, 142 patients were divided into a monosensitized group and a polysensitized group. We found that both total IgE and unknown IgE were higher in the polysensitized group than in the monosensitized group (Figure 4A). Similar to the results of the family allergy history analysis, Der f and Der p sIgE levels showed no differences between the two groups (Figure 4B). However, the ratio of Der f sIgE/tIgE and Der p sIgE/tIgE were higher in the monosensitized group than in the polysensitized group (Figure 4C). This result suggests that polysensitivity may affect the level of total IgE and the ratio of sIgE/tIgE.

Discussion
The HDM is the most prevalent allergen in patients with asthma and/or rhinitis in China (17). In this study, asthmatic children with dust mite SPTs of “+++” or above were enrolled. Among the 142 child patients allergic to dust mites, 93 were male (65.49%). Sex-related differences in asthma prevalence are well established and change through the reproductive phases of life. As children, boys have an increased prevalence of asthma compared to girls. Atopic asthma prevalence during childhood is typically higher in males compared to females (18-20). In this regard, our study is consistent with previous studies. Family history of allergy or history of asthma strongly influences asthma risk in children, as London et al., have shown that parental history of asthma and allergy is most strongly associated with early onset persistent asthma (21). Another study revealed that a family history of asthma and allergic diseases is a strong determinant of asthma (22). In our study, 58.45% of patients had a family history of allergy or asthma, which further supports the role of family history in the development of allergic asthma. AR and asthma are common diseases that frequently occur together, and, as they are both IgE-mediated diseases, they are triggered by similar allergens and have interrelated inflammatory and pathophysiological mechanisms. In our study, 92.95% of the allergic asthma patients suffered from concomitant AR. In fact, the co-occurrence of AR and asthma has been defined as combined allergic rhinitis and asthma syndrome (CARAS), and fits with the “one airway, one disease” concept of this condition. AR and asthma are characterized by upper and lower airway inflammation, respectively, with the genesis of both diseases being similar. As they are triggered by the same etiological agents, these phenomena co-occur, have the same inflammatory cell profile, and share the same therapeutic treatment (23).
We found that the positive rates of Der p, Der f, and Blo t were 100%, 100%, and 91.84% respectively, while the positive rates of other inhalation allergens such as cat dander, dog dander, and cockroach were all lower than 20%. Therefore, dust mites are the main allergen in children with allergic asthma. Meanwhile, the allergens affecting asthma patients can also include food allergens, such as egg white, milk, shrimp, and crab, which are the most common food allergies in children. Unfortunately, we did not measure sIgE for pollens, and we will complete pollen-specific IgEs detection in future studies. It is reported that IgE contains allergen-specific IgE, virus-specific IgE, bacteria-specific IgE (24), and allergen-specific IgE which can be detected, and so unknown IgE contains virus-specific IgE, bacteria-specific IgE, and some allergen-specific IgE which cannot be detected by current testing methods. However, because of the limitations of technology, the exact components of unknown IgE remain unclear. We found that both monosensitized and polysensitized patients, unknown IgE accounted for more than 60% of the tIgE, So the study for the exact components of unknown IgE may be beneficial for asthma, and that may be the direction of future efforts.
Our study also found that the level of total IgE had a close correlation with the level of Der p and Der f sIgE. This is in line with reports from other studies (25-26). Furthermore, sIgE levels of Der f and Der p, along with serum IgE for Der p1 and Der p2 and the serum IgE for Der p were also highly correlated. Our study suggests that for the detection of Der p1 or Der p2, sIgE may be a suitable tool with sufficient diagnostic accuracy for use in the diagnosis of Der-p IgE sensitization. This was also found in a report from Central China (25). More importantly, the close correlation between serum IgE titers and Der p 1 and Der p 2 is in line with reports by Wang et al. in Easter China (26).
We further found that the asthmatic patients with a family history of allergy had a higher total IgE and unknown IgE than those patients without a family history of allergy. As described above, unknown IgE contains virus-specific IgE, bacteria-specific IgE, and some allergen-specific IgE which cannot be detected by the current testing methods. This suggests that asthmatic patients with a family history of allergy are more susceptible to viruses and bacteria. As mentioned in the introduction above, the serum tIgE and sIgE/tIgE ratio can be used as a predictor of AIT efficacy. A significant correlation was found between the serum sIgE/tIgE ratio and the clinical response to AIT, with higher ratios (>16.2) being associated with an effective response (13). Another study reported that tobacco smoke exposure, a family history of atopic disease, serum tIgE ≥965 kU/l, and a sIgE/tIgE ratio ≥6% were associated with an ineffective clinical response to AIT in children, which may be helpful for patient selection before immunotherapy. The same study also concluded that serum tIgE is superior to both the serum sIgE/tIgE ratio and sIgE levels alone in predicting clinical effectiveness (14). Sun et al. showed that tIgE lower than 610 kU/L and sIgE/tIgE ratio greater than 15.0% have the best sensitivity and specificity for predicting a valid response to AIT (15). Vidal and his colleagues found that use of allergen-specific IgE levels >9.74 kUA/L as a biomarker of effective AIT yielded a sensitivity value of 96.4% and a specificity of 100% (16). Therefore, for patients with allergic asthma, the molecular characteristics of IgE (total IgE, specific IgE, sIgE/tIgE, polysensitized or monosensitized) and a family history of allergy may play an important role in predicting the effectiveness of AIT. In our study, asthmatic patients with a family history of allergy had a higher total IgE and a lower ratio of Der f sIgE/tIgE and Der p sIgE/tIgE. We can thus extrapolate that asthmatic patients without a family history of allergy may benefit more from AIT. Our study also divided patients into a polysensitized group and a monosensitized group, and we found that the polysensitized group had higher total IgE and unknown IgE levels than the monosensitized group. The polysensitized group also had a lower the ratio of Der f sIgE/tIgE and Der p sIgE/tIgE, meaning that the monosensitized group may benefit more from AIT.
Conclusions
In summary, this study was the first to analyze and report the IgE molecular sensitization profile of allergic asthma among children in China. We found that dust mites are the main allergen in children with allergic asthma. Our findings indicate patients with no family history of allergy and monosensitized patients have a higher ratio of sIgE/tIgE, and those patients may benefit more from AIT.
Acknowledgments
The authors appreciate the academic support from AME Allergic Asthma Collaborative Group.
Funding: This study was sponsored by National Clinical Research Center for Child Health and Disorders (Grant No.: NCRC-2019-GP-06) and Chongqing Municipal Science and Technology Bureau (The central government guides development of local science and technology fund).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-7314
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-7314
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-7314). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University (file no. 2018-02), and each participant’s statutory guardian signed the informed consent. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global strategy for asthma management and prevention, updated 2020.
- Thirteenth General Programme of Work, 2019-2023. Geneva: World Health Organization, 2018.
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18. [Crossref] [PubMed]
- Chinese Pediatric Asthma Prevention and Coordination Group. Prevalence of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi 2003;41:123-7.
- Chinese Pediatric Asthma Prevention and Coordination Group. The third epidemiological survey of childhood asthma in Chinese cities. Zhonghua Er Ke Za Zhi 2013;51:729-35. [PubMed]
- Wang J, Wu Y, Li J, et al. Eight aeroallergen skin extracts may be the optimal panel for allergic rhinitis patients in central China. Int Arch Allergy Immunol 2017;173:193-8. [Crossref] [PubMed]
- Muraro A, Lemanske RF, Hellings PW, et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academyof Allergy, Asthma & Immunology. J Allergy Clin Immunol 2016;137:1347-58. [Crossref] [PubMed]
- Lai T, Wang S, Xu Z, et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep 2015;5:8191. [Crossref] [PubMed]
- Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017;72:1825-48. [Crossref] [PubMed]
- Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med 1999;341:468-75. [Crossref] [PubMed]
- Virchow JC, Backer V, Kuna P, et al. Efficacy of a House Dust Mite Sublingual Allergen Immunotherapy Tablet in Adults with Allergic Asthma A Randomized Clinical Trial. JAMA 2016;315:1715-25. [Crossref] [PubMed]
- Canonica GW, Bachert C, Hellings P, et al. Allergen Immunotherapy (AIT): a prototype of Precision Medicine. World Allergy Organ J 2015;8:31. [Crossref] [PubMed]
- Di Lorenzo G, Mansueto P, Pacor ML, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol 2009;123:1103-10. [Crossref] [PubMed]
- Li Q, Li M, Yue W, et al. Predictive Factors for Clinical Response to Allergy Immunotherapy in Children with Asthma and Rhinitis. Int Arch Allergy Immunol 2014;164:210-7. [Crossref] [PubMed]
- Sun W, Pan L, Yu Q, et al. The Skin Prick Test Response after Allergen Immunotherapy in Differen Levels of tIgE Children with Mite Sensitive Asthma/Rhinitis in South China. Hum Vaccin Immunother 2018;14:2510-5. [Crossref] [PubMed]
- Vidal C, Enrique E, Gonzalo A, et al. Diagnosis and allergen immunotherapy treatment of polysensitised patients with respiratory allergy in Spain: an Allergists’ Consensus. Clin Transl Allergy 2014;4:36. [Crossref] [PubMed]
- Li J, Sun B, Huang Y, et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy 2009;64:1083-92. [Crossref] [PubMed]
- Lichtenstein P, Svartengren M. Genes, environments, and sex: factors of importance in atopic diseases in 7-9-year-old Swedish twins. Allergy 1997;52:1079-86. [Crossref] [PubMed]
- de Marco R, Locatelli F, Sunyer J, et al. Differences in Incidence of Reported Asthma Related to Age in Men and Women. Am. J. Respir. Crit. Care Med 2000;162:68-74. [Crossref] [PubMed]
- Wijga A, Tabak C, Postma DS, et al. Sex differences in asthma during the first 8 years of life: The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study. J Allergy Clin Immunol 2011;127:275-7. [Crossref] [PubMed]
- London SJ, Gauderman WJ, Avol E, et al. Family history and the risk of early onset persistent, early onset transient and late onset asthma. Epidemiology 2001;12:577-83. [Crossref] [PubMed]
- Paaso EMS, Jaakkola MS, Rantala AK, et al. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respir Res 2014;15:152. [Crossref] [PubMed]
- Paiva Ferreira LKD, Paiva Ferreira LAM, Monteiro TM, et al. Combined allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol 2019;74:105718. [Crossref] [PubMed]
- Froidure A, Mouthuy J, Durham SR, et al. Asthma phenotypes and IgE responses. Eur Respir J 2016;47:304-19. [Crossref] [PubMed]
- Yang Y, Zhu R, Huang N, et al. The Dermatophagoides pteronyssinus Molecular Sensitization Profile of Allergic Rhinitis Patients in Central China. Am J Rhinol Allergy 2018;32:397-403. [Crossref] [PubMed]
- Wang HY, Gao ZS, Zhou X, et al. Evaluation of the Role of IgE Responses to Der p 1 and Der p 2 in Chinese House Dust Mite-Allergic Patients. Int Arch Allergy Immunol 2015;167:203-10. [Crossref] [PubMed]
(English Language Editor: J. Gray)


