
This article has an erratum available at: http://dx.doi.org/10.21037/atm-2023-11 the article has been update on 2023-05-22 at here.
Evaluation of factors affecting the visualization of dye after transbronchial dye injection: an animal experiment
Introduction
Since peripheral lung lesions located more than 1 cm deep within the visceral pleura can be frequently visualized on computed tomography (CT), various methods of localization for thoracoscopic surgery have been developed, such as transthoracic needle localization or dye injection through bronchoscopy (1-7). With the methods that use transthoracic percutaneous localization, the occurrence of post-procedural complications, such as pneumothorax or bleeding, is possible (1,3,6). In the case of transbronchial dye injection (TDI) via bronchoscopy, the aforementioned complications do not occur based on several articles (7,8); however, there can be difficulties with dye visualization through the thoracoscope depending on early disappearance of the dye due to diffusion before field exposure (1,8-10).
Herein, we performed an animal experiment to determine how much dye is needed for visualization of peripheral lung lesions, how long the dye lasts, when the dye disappears, and whether air injection following dye injection is necessary. We present the following study in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-1695).
Methods
Animal model and study groups
Twelve live female farm pigs were used to identify the appropriate amount of indigo carmine needed for localization of peripheral lung lesions using a bronchoscope. The mean age of the pigs was 11.2±1.2 (range, 10–13) weeks, and weight was 39.3±1 (range, 38.4–41.1) kg. Experiments were performed under a project license (No. PNUYH-2018-059) granted by the Institutional Animal Care and Use Committee of Pusan National University Yangsan Hospital in compliance with Pusan national university guidelines for the care and use of animals.
Nine pigs were categorized into three groups based on the amount of dye injected: group 1 received 0.6 mL of dye (n=3); group 2 received 0.8 mL of dye (n=3); and group 3 received 1.0 mL of dye (n=3), all followed by 2.0 ml of air injection. Another three pigs were included in group 4, which received 1.0 mL of dye followed by no air injection to evaluate the utility of air injection.
Generally, TDI was clinically conducted to detect peripheral lung lesion within 2 cm from visceral pleura, the location of injection was targeted to the area within 1 cm from visceral pleura nearby the lung lesion for the indigo carmine to be easily detected on visceral pleura.
Animal experiment
All procedures were performed with the pigs under general anesthesia using an intravenous injection of propofol (4–12 mg/kg/h) and intramuscular injection of tiletamine and zolazepam (4.4–6.6 mg/kg), and meloxicam (0.4 mg/kg). Orotracheal intubation was performed initially, followed by tracheostomy. Under supine position, the predicted place for the thoracoscopic port was generally incised at the sixth mid-axillary line and seventh posterior axillary line of the intercostal space before bronchoscopic dye administration, and dissected before reaching the parietal pleura, which was cautiously not punctured to prevent pneumothorax.
Before bronchoscopy, a guide sheath (K-201; Olympus, Tokyo, Japan) and biopsy forceps were prepared so the surgeon could approach the peripheral region of the porcine lung. The bronchoscope (BF-260; Olympus, Tokyo, Japan) was advanced as far as possible under direct vision to superior segment of right lower lobe; thereafter, guide sheath-covered biopsy forceps were introduced through the working channel of the bronchoscope under fluoroscopic guidance. When the guide sheath-covered biopsy forceps reached within 1cm from visceral pleura under fluoroscopic guidance, the biopsy forceps were removed, while the guide sheath was kept in place for subsequent administration of indigo carmine (Daiichi-Sankyo Inc., Tokyo, Japan). To maintain one-lung ventilation during thoracoscopic inspection, the endotracheal tube was inserted into the tracheostomy orifice and placed in the left main bronchus under bronchoscopic guidance (BF-P160; Olympus). Just after TDI, the pigs were placed in lateral decubitus position and followed by inserting two ports. The thoracoscope was advanced to examine the dyed area of the visceral pleura.
Definitions of the measured times
The detection time was defined as when the dyed area of the visceral pleura was identified for the first time. The peak time was defined as when the dyed area was fully extended. The instrument was used for measuring, for example, fully opening of the tip clinch was measured as 2.5 cm. The wash-out time was defined as when the dyed extent of the visceral pleura became less than two-third of the peak time.
Statistical analysis
The t-test and one-way analysis of variance were used to compare data between the study groups for statistical significance. The data are expressed as the mean ± standard deviation (range). A P value <0.05 was considered as statistically significant. All data analyses were conducted using SPSS Statistics software, version 24.0 (IBM, Corp., Armonk, NY, USA).
Results
The mean age of the pigs was 11.2±1.2 (range, 10–13) weeks, and weight was 39.3±1 (range, 38.4–41.1) kg. TDI was performed in 12 pigs (three pigs in each group), and the most common injected site was the superior segment. The mean bronchoscopy time was 2.4±0.48 (range, 1.8–3.4) minutes, and fluoroscopy time was 2.13±0.72 (range, 1.4–3.7) minutes. All the pigs were alive and had not shown the unstable vital sign during the procedure, and the procedure was successfully performed.
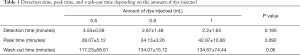
The mean detection times for 0.6, 0.8, and 1 mL of dye were 4.53±0.59 (range, 4.1–5.2), 2.67±1.48 (range, 1.4–4.3), and 2.2±1.65 (range, 1.2–4.1) minutes, respectively, which were not significantly different (P=0.195) (Figure 1). The mean peak times for 0.6, 0.8, and 1 mL of dye were 28.07±5.12 (range, 23.2–33.4), 34.13±3.26 (range, 30.4–36.4), and 42.97±10.86 (range, 35.3–55.4) minutes, respectively, which were not significantly different (P=0.092) (Figure 1). The mean wash-out times for 0.6, 0.8, and 1 mL of dye were 117.23±68.61 (range, 75.1–196.4), 134.07±15.72 (range, 119.3–150.6), and 134.67±74.44 (range, 85.4–220.3) minutes, respectively, which were not significantly different (P=0.06) (Table 1) (Figure 1).
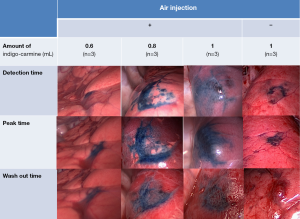
Full table
In group 4, the mean detection time, mean peak time, and mean wash-out time were 3.27±1.90 (range, 1.4–5.2), 12.1±1.6 (range, 10.4–13.6), and 46.23±10.58 (range, 38.1–58.2) minutes, respectively. In group 3, those respective times were 2.2±1.65 (range, 1.2–4.1), 28.07±5.12 (range, 23.2–33.4), and 134.07±15.72 (range, 119.3–150.6) minutes. The peak time and wash-out time were statistically significantly different between groups 3 and 4 (P=0.07 and 0.001, respectively) (Table 2).

Full table
Measured sizes of the dye extent at the detection time, peak time, and wash-out time
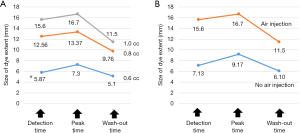
The mean measured sizes of 0.6, 0.8, and 1.0 mL of dye extent at the detection time, peak time, and wash-out time were significantly different (P=0.001, 0.001, and 0.002, respectively) (Table 3). The mean measured sizes of 0.6, 0.8, and 1 mL of dye extent at the detection time, peak time, and washed-out time were significantly different between groups 3 and 4 (P=0.003, 0.003, and 0.001, respectively) (Figure 2, Table 4). The change in the size of the dye extent was significant over time (P<0.001), and the size of the dye extent was significantly different depending on the amount of dye injected (P=0.004). And group 2 has significant difference in detection time, peak time and wash-out time comparing with group 1 (P=0.06, 0.009, 0.011, respectively). The change in the size of dye extent was significantly changed over time in group 4 compared with group 3 (P<0.001), and the size of the dye extent was significantly smaller in group 4 than in group 3 (P<0.001).

Full table

Full table
In summary, the mean detection times, the mean peak times, and the mean wash-out times for 0.6, 0.8, and 1.0 mL of dye were not significantly different each other. However, between groups 3 and 4, the peak time and wash-out time were statistically significantly different (P=0.003 and 0.001, respectively), which meant the application of air injection might be important to be exposed longer time.
Discussion
Indeterminate pulmonary nodules have been frequently detected since chest CT has been developed. In cases with an increasing number of nodules, therapeutic or diagnostic resection needs to be considered. Although CT-assisted percutaneous marking using lipiodol or wire might be most commonly used for non-palpable nodules located more than 1 cm deep within the visceral pleura, pneumothorax could occur in about 15% of cases, and lipiodol could be spilled into pleural cavity, which might cause pleuritic chest pain and the wire could become dislodged (3,6,10-14). With lipiodol marking, exposure of radiation cannot be avoidable for the patient and operating team (12-14).
TDI using indigo carmine might be another option to make small nodules identifiable by direct vision (1,8-10). Clinically, TDI has been frequently performed in these days, which let the risk of complications decreased, such as pneumothorax, hemothorax, and pleuritic chest pain. However, the dye can be easily and rapidly diffused (8-10), making it potentially difficult to identify the nodule as the interval time between TDI and exploration under video-assisted thoracoscopic surgery becomes longer. Especially if a patient has severe adhesion, the injected dye could become diffused and spontaneously resolve during dissection of the adhesion, and multiple areas of hemorrhage or anthracosis mimicking the color of the dye could cause confusion (8,9).
In our series, there were no significant differences in the detection time, peak time, and wash-out time among groups with 0.6, 0.8, and 1.0 mL of dye injected. However, in the group without air injection, the detection time was significantly delayed, and the interval time to the peak time and wash-out time was significantly shorter than that in the group with air injection. This finding might explain why remnant dye could be left in the catheter even if the same amount of dye was injected. Since air injection after dye injection enabled the dye to be completely sprayed through the catheter, indigo carmine could be easily identified on the peripheral lung parenchyma. As shown in the results, the marking could be identified clearly at about 2 hours after TDI regardless of the amount of indigo carmine injected. Without air injection, the marking might only last for 1 hour.
In our study, the higher the amount of dye injected, the larger the dye extent was visualized, and the dye could be more easily detected in the group with air injection than in the group without air injection group. To compare the difference in size of dye extent among each group, group 2 has significant difference comparing with group 1 (P<0.05) (Figure 2). Thus, in cases in which (I) 2 hours has passed after injection of (II) less than 0.8 mL of dye or (III) no air injection, visualization of dye might fail during thoracoscopic surgery.
Still, this study has some limitations. First, it is a small-sized experiment, which does not have strong statistical power. Second, it is performed by one of experts in bronchoscopic intervention, which means it cannot guarantee same result in case by the other specialist. Third, although the pigs are relatively suited to human based on similarities in their respiratory system, it could be mistranslated to human biology.
Conclusions
TDI might be reliable and efficient for detecting small peripheral lung nodules in cases with more than 0.8 mL of dye injected followed by air injection within 2 hours after dye injection. However, in cases with severe adhesion and anthracotic pigmentation, it makes longer predicted time to lesion exposure or confusing the lesion, and also for emphysema, it could be diffused easily which don’t let the injected area be localized. Therefore, it might be necessary to discover the mixture of dye which will last longer for visualization of lung nodules and distinct from anthracotic pigmentation.
Acknowledgments
This article was presented as poster in “2019 Chest congress”.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1005004).
Footnote
Reporting Checklist: The authors present the study in accordance with the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-1695
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-1695
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-1695). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. PNUYH-2018-059) granted by the Institutional Animal Care and Use Committee of Pusan National University Yangsan Hospital in compliance with Pusan national university guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Endo M, Kotani Y, Satouchi M, et al. CT fluoroscopy-guided bronchoscopic dye marking for resection of small peripheral pulmonary nodules. Chest 2004;125:1747-52. [Crossref] [PubMed]
- Keating J, Singhal S. Novel methods of intraoperative localization and margin assessment of pulmonary nodules. Semin Thorac Cardiovasc Surg 2016;28:127-36. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Kha LC, Hanneman K, Donahoe L, et al. Safety and efficacy of modified preoperative lung nodule microcoil localization without pleural marking: a pilot study. J Thorac Imaging 2016;31:15-22. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Olaiya B, Gilliland CA, Force SD, et al. Preoperative computed tomography-guided pulmonary lesion marking in preparation for fluoroscopic wedge resection-rates of success, complications, and pathology outcomes. Curr Probl Diagn Radiol 2019;48:27-31. [Crossref] [PubMed]
- Silvestri GA, Herth FJ, Keast T, et al. Feasibility and safety of bronchoscopic transparenchymal nodule access in canines: a new real-time image-guided approach to lung lesions. Chest 2014;145:833-8. [Crossref] [PubMed]
- Krimsky WS, Minnich DJ, Cattaneo SM, et al. Thoracoscopic detection of occult indeterminate pulmonary nodules using bronchoscopic pleural dye marking. J Community Hosp Intern Med Perspect 2014. [Crossref] [PubMed]
- Hasegawa T, Kuroda H, Sato Y, et al. The utility of indigo carmine and lipiodol mixture for preoperative pulmonary nodule localization before video-assisted thoracic surgery. J Vasc Interv Radiol 2019;30:446-52. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. [Crossref] [PubMed]
- Jin KN, Lee KW, Kim TJ, et al. Computed tomography guided percutaneous injection of a mixture of lipiodol and methylene blue in rabbit lungs: evaluation of localization ability for video-assisted thoracoscopic surgery. J Korean Med Sci 2014;29:129-36. [Crossref] [PubMed]
- Miura H, Yamagami T, Tanaka O, et al. CT findings after lipiodol marking performed before video-assisted thoracoscopic surgery for small pulmonary nodules. Acta Radiol 2016;57:303-10. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]


