
Predictive value of EGF and uPAR for chemoradiotherapy response and survival in patients with esophageal squamous cell carcinoma
Introduction
Over the past decades, esophageal carcinoma is a leading cause of death in cancer patients worldwide. Esophageal squamous cell carcinoma (ESCC) accounts for most esophageal malignant tumors in Eastern Europe and Asia, and the 5-year overall survival rarely exceeds 30% (1-3). Neoadjuvant chemoradiotherapy (nCRT) plus surgery has recently become the standard therapeutic strategy for locally advanced ESCC, with significant survival benefit compared with surgery alone (4,5). However, the response of individual tumors to nCRT was highly variable. Some studies have attempted to accurately assess CRT responses with different diagnostic approaches, but the results have yet been mostly unsatisfactory (6,7). Therefore, it is urgently needed to find reliable and effective biomarkers to predict CRT sensitivity and prognosis of ESCCs to promote individualized treatment.
Cytokines are a group of small soluble proteins with low molecular weight that play important roles in the control of the communication between cancerous cells, stromal cells and immune cells in tumor microenvironment (8,9). As essential intercellular mediators, they promote cell growth and participate in their differentiation, migration and apoptosis (8). Although cytokines in some cases contribute to support of host anti-tumor response, evidence accumulated over the past decade suggested that cytokine networks have been involved in promoting tumor progression, including tumor growth, invasion, metastasis, and even host immunosuppression (10,11). Previous studies indicated that cytokines have been considered as significantly prognostically and associated with clinical and pathological changes in ESCC (12,13). However, the expression level of serum cytokines varies person to person and the optimal serum cytokines level for predicting survival is contentious, but variabilities in the level of cytokines before and after treatment are relatively stable for each person. The alterations of serum cytokine expression after CRT remains unstudied, and little is known about their significance in ESCC prognosis.
Therefore, our goal here was to determine whether alterations of serum cytokine levels after CRT could be predictive of disease prognosis in ESCC patients. To this end, a panel of 120 known tumor related cytokines were measured in pretreatment serum compared with serum levels after CRT. We have identified up-regulation of EGF and uPAR to be significantly associated with adverse clinical outcomes. In present study, changes in serum EGF and uPAR expression were examined in a group of 68 ESCC patients, and the predictive value of EGF and uPAR for patient survival was further investigated. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4503).
Methods
Study populations
This pilot study enrolled patients who received neo-adjuvant CRT (weekly cisplatin and docetaxel chemotherapy for four cycles combined with radiotherapy with a dose of 40 Gy in 20 fractions) followed by radical surgery 4 to 6 weeks later or definitive CRT (weekly cisplatin and docetaxel chemotherapy for four cycles combined with radiotherapy with a dose of 60 Gy in 30 fractions) between 2015 and 2017. Informed consent was obtained from each patient to undergo blood samples collection before treatment and at the time of administration of total doses of 40 Gy. All patients had undergone a standardized staging evaluation, including upper gastrointestinal endoscopic ultrasonography with biopsies, the neck, chest, and abdomen computed tomography (CT) scanning, and external ultrasonography of the neck, and the disease stage was identified according to the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. The study was approved by the medical ethics committee of the institute. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Treated patients met the following inclusion criteria: (I) diagnosis of ESCC based on pathologic evaluation; (II) Karnofsky performance status of 70 or higher; (III) untreated patients who have not received any antitumor therapy, including chemotherapy, radiotherapy or surgery; (IV) life expectancy at least 3 months following CRT. Patients were excluded if they had a history of other malignancy, had not completed CRT, or had evidence of distant metastasis.
Serum examples collection and cytokine detection
Blood samples were collected before treatment and at the time of administration of total doses of 40 Gy. Venous blood samples were collected in serum tubes and allowed to clot. After centrifugation, serum was collected, aliquoted, and stored at −80 °C until analysis. Simultaneous detection of multiple cytokines undoubtedly provides a powerful tool to study cytokines. We detected serum concentrations of 120 known tumor-related cytokines by the human cytokine antibody array (RayBio Human Cytokine Antibody Array G series 1000, Raybiotech, Norcross GA, USA) which is a glass slide that is a highly sensitive approach to simultaneously detect multiple cytokine expression levels from diverse sample types.
ALL 8 patients were males, and had a higher percentage of ≤60 years (62.5%). Two patients underwent surgical esophageal resection. Five tumors were stage III, three stage IVA. Seven of them, lesions were located in the middle part of the esophagus. Eight patients in screening group were categorized into two group based on their CRT response. CRT response was evaluated clinically for primary lesions based on CT, endoscopic ultrasonography and esophagus biopsy when the total tumor doses were 40 Gy. Patients achieving complete response or partial response were divided into CRT sensitivity group, and patients achieving stable disease or progressive disease were divided into CRT resistance group. Differentially expressed cytokines were screened by cytokine antibody arrays in pre- and post-treatment serum from 4 patients with CRT sensitivity and 4 patients with CRT resistance. Subsequently, the prognostic value of differentially expressed cytokines was further validated in a second set of 60 ESCCs. Two patients did not detect pre-treatment uPAR expression because of insufficient serum samples and three patients (including one female) did not detect EGF expression before and after treatment because of insufficient serum samples.
Follow-up and statistical analysis
Progression-free survival was defined as the time from diagnosis of ESCC to first locoregional or distant recurrence. Overall survival was the time from ESCC diagnosis to cancer-relative death. Two patients were lost to follow-up. Weight loss was defined as >5% unintentional weight loss during the 3 months before disease diagnosis. Changes in uPAR expression level before and after CRT were defined as uPAR radio = post-treatment uPAR expression level/pretreatment uPAR expression level, and changes in EGF expression level before and after CRT were defined as EGF radio = post-treatment EGF expression level/pretreatment EGF expression level. Pearson correlation coefficient analysis was performed to investigate the correlation between EGF and uPAR expression in serum. We conducted univariate and multivariate analyses using the Kaplan–Meier method and the Cox proportional hazards model to assess factors related to patients’ survival. Potential risk factors with a P value of <0.1 in the univariate analysis were entered into a multivariate analysis to determine their independent effect. All statistical analyses were performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA) and Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA). A P value <0.05 was considered statistically significant from two-sided tests.
Results
Baseline characteristics of patients
A total of 68 patients met the study criteria and were fully evaluated. Blood specimens were obtained pre-treatment and during treatment for each patient. Of the 68 patients, 60 (88.2%) were men, and 54 (79.4) had ever smoked. The median patient age was 60 years (range, 47–79 years). Baseline patient characteristics are detailed in Table 1.
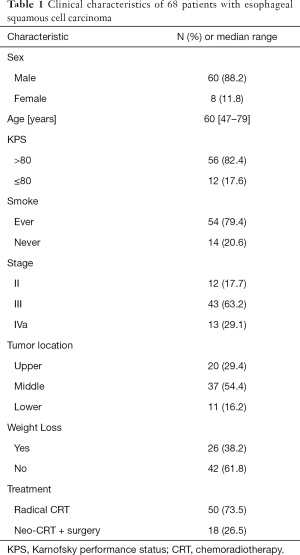
Full table
Screening of serum cytokines in ESCC patients
In our study, to investigate the correlation between serum cytokine changes and clinical outcomes in ESCC, cytokine microarrays containing 120 human cytokines were performed to detect the expression of serum cytokines before and after CRT from 8 ESCC patients (Figure 1). With the 2-fold cutoff point, 7 differentially expressed cytokines were validated in 4 patients with favorable prognosis and 4 patients with adverse prognosis (EGF, uPAR, MIP-1β, MIF, IL-8, PDGF-BB and BDNF). Of these, up-regulation of EGF and uPAR in serum after CRT were associated with a poor response to CRT. The predictive value of uPAR and EGF were further assessed in a second set of 60 ESCCs. Consistently, elevated EGF and uPAR expression were strongly associated with worse clinical outcomes after CRT.

Prognostic significance of serum EGF and uPAR levels in ESCC patients
The median follow-up period was 15.87 months (range, 6.21–23.85 months). At last follow-up, 23 patients (34.9%) had died with disease progression, 15 (22.7%) were alive with disease progression, and 28 (42.4%) were alive without progression.
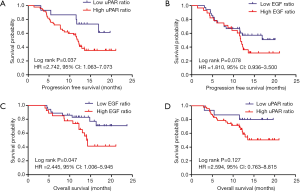
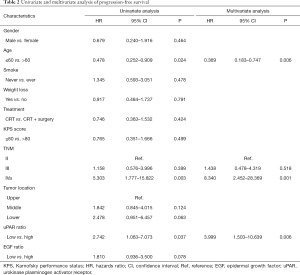
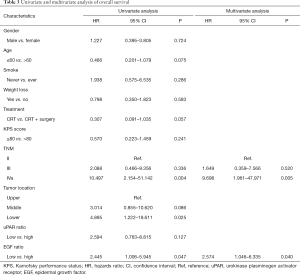
The high uPAR ratio after CRT correlated closely with inferior PFS (Figure 2A; HR =2.742, 95% CI: 1.063–7.073, P=0.037), while EGF ratio was not associated with PFS (Figure 2B). Univariate analysis revealed that age, TNM stage, and high uPAR ratio were associated with PFS. Moreover, from the further multivariate analysis, age, TNM stage and high uPAR ratio remained the independent predictors of PFS (Table 2). Similarly, increased EGF expression after CRT were positively correlated with adverse OS (Figure 2C; HR =2.445, 95% CI: 1.006–5.945, P=0.047), while uPAR ratio showed no predictive significance (Figure 2D). Univariate and multivariate analysis showed that TNM stage and high EGF ratio were independent predictors of OS (Table 3).
Full table
Full table
When the ESCCs patients were separated into two groups, the 26 patients with both high EGF and uPAR ratios had a worse PFS and OS, compared to the 37 patients with a high EGF ratio only or uPAR ratio only or neither (1-year PFS rate 44.2% vs. 61.4%, 1-year OS rate 64.2% vs. 83.4%, P=0.033 and 0.029, respectively). Representative Kaplan-Meier survival curves based on these factors are shown in Figure 3A,B.

Correlation between the expression level of uPAR and EGF in patients with ESCC
Given the emerging perspective that uPAR and EGF are cytokines with some interacting signaling pathways, we postulated that there may be a correlation between levels of uPAR and EGF in serum. A correlation analysis revealed that uPAR expression was positive correlation with EGF before treatment (r=0.4884, P=0.0001; Figure 4A). Similarly, significantly positive correlation was also observed between uPAR and EGF in posttreatment serum (r=0.3775, P=0.0038; Figure 4B).

Discussion
Chemoradiotherapy (CRT) has been commonly used for the therapeutic strategy for ESCC, however, effective and reliable biomarkers predicting CRT response and prognosis have yet to be fully elucidated. Although accumulating studies have shown that cytokines present within esophageal cancer contribute not only to tumor proliferation and angiogenesis, but also to the metastasis and survival of patients (14-16), to our knowledge, this is the first study using a high-throughput cytokine microarray which containing 120 known tumor-related cytokines, to identify novel and effective biomarkers for predicting prognosis in ESCC patients receiving CRT. Our study demonstrated that up-regulated uPAR and EGF cytokines after CRT are positively associated with poor PFS and shortened survival.
Epidermal growth factor (EGF) and its receptor play a key role in in regulating the cell cycle, apoptosis, cell proliferation, differentiation, as well as malignant transformation and progression in a variety of normal and malignant cells (17). Previously, a study of metastatic models in human esophageal cancer cell lines reported that EGF can induce epithelial-mesenchymal transition (EMT) which was the hallmark of tumor metastasis (18). Moreover, similar results, were also observed in human breast carcinoma cell and gastric cancer cells, which indicated EGF contribute to several type cancers progression (19,20). However, some reports have shown contradictory results of EGF in tumor development and progression (21,22). To date, studies of alterations to EGF levels in serum after CRT remain elusive, thus little is known about their prognostic value in patients with ESCC. It is widely known that the signaling networks of EGF/EGFR are complicated and interwoven with each other (17). Early studies demonstrated that the expression of uPAR noticeably up-regulated after EGF stimulation through the activation of Arf6/ERK/uPAR signaling pathway, resulting in the aggressiveness of breast cancer cells (23). A similar observation was made by Wang et al. in which EGF promoted gastric cancer cell invasion via activating the ERK1/2 pathway and, subsequently, leading to the up-regulation of uPAR (20). Mounting evidence indicated that EGF/EGFR signaling network served as a pivotal role in the regulation of uPAR. The urokinase plasminogen activator receptor (uPAR/CD87), a cell-surface glycoprotein consisting of three homologous cysteine-rich domains, was recently evaluated as a prognostic biomarker in human cancers (24-27). Previous research reported expression of uPAR was strongly correlated with clinical tumor stage, lymph nodes, and metastases in urinary bladder carcinoma (28). Similarly, Memarzadeh et al. has demonstrated that uPAR was appear to be an independent prognostic indicator for endometrial cancer (29). Consistent with these studies, our findings showed that high serum levels of EGF and uPAR were closely associated with adverse outcomes in patient with ESCC. Subsequently, our study revealed a significantly positive correlation between EGF and uPAR, which may suggest that EGF and uPAR may be involved in some same signaling pathways and play synergistic roles in cancer progression.
A growing body of evidence have showed that elevated levels of uPAR and EGF, which were considered to be biological markers, have been clearly demonstrated to be essential for maintaining tumor aggressiveness and promoting metastasis (30-32). The present study for the first time revealed that patients with higher levels of EGF and uPAR may be more resistant to CRT, thus resulting in adverse clinical outcome. Moreover, our study suggested that collectively examination of these candidate biomarkers should be developed as a novel and promising prognostic model in ESCC patients. However, some limitations deserve mention in this study. There are only 68 ESCCs and patients had a short duration of follow-up. Additional clinical studies with a larger sample of patients are warranted to validate our findings. In addition, basic works are needed to elucidate the role of EGF and uPAR in tumor progression and to clarify the underlying mechanisms by which cytokines regulate ESCC responses to CRT.
Conclusions
In summary, this study was conducted to evaluate the effects of CRT on EGF and uPAR expression levels and evaluate possible clinical implications for patients with esophageal cancer. Moreover, multivariate analysis revealed that CRT-induced alterations of EGF and uPAR levels are valuable and promising predictors of survival. Additionally, these data may provide the rationale for multipronged approaches to combine CRT with molecular targets in patients with ESCC, with the goal of overcoming CRT resistance to improve survival.
Acknowledgments
Funding: This study was supported by the National Nature Science Foundation of China (No. 81602565, 81872462), the Clinical Trial supporting Foundation of Tianjin Medical University Cancer Institute & Hospital (Grant No. C1707) and the State Key Laboratory of Medicinal Chemical Biology (Nankai University) (No. 2019012).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4503
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4503
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4503). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the medical ethics committee of the institute (No. E2016103A). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Semenkovich TR, Meyers BF. Surveillance versus esophagectomy in esophageal cancer patients with a clinical complete response after induction chemoradiation. Ann Transl Med 2018;6:81. [Crossref] [PubMed]
- Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 2004;78:1152-60. [Crossref] [PubMed]
- Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965-74. [Crossref] [PubMed]
- Wilson J, Balkwill F. The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol 2002;12:113-20. [Crossref] [PubMed]
- Eiró N, Vizoso FJ. Inflammation and cancer. World J Gastrointest Surg 2012;4:62-72. [Crossref] [PubMed]
- Liu J, Li Z, Cui J, et al. Cellular changes in the tumor microenvironment of human esophageal squamous cell carcinomas. Tumour Biol 2012;33:495-505. [Crossref] [PubMed]
- Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev 2006;17:141-6. [Crossref] [PubMed]
- Ichiba M, Miyazaki Y, Kitamura S, et al. Epidermal growth factor inhibits the growth of TE8 esophageal cancer cells through the activation of STAT1. J Gastroenterol 2002;37:497-503. [Crossref] [PubMed]
- Diakowska D. Cytokines association with clinical and pathological changes in esophageal squamous cell carcinoma. Dis Markers 2013;35:883-93. [Crossref] [PubMed]
- Chung Y, Law S, Kwong DL, et al. Serum soluble E-cadherin is a potential prognostic marker in esophageal squamous cell carcinoma. Dis Esophagus 2011;24:49-55. [Crossref] [PubMed]
- Cheng JC, Graber MS, Hsu FM, et al. High serum levels of vascular endothelial growth factor-A and transforming growth factor-β1 before neoadjuvant chemoradiotherapy predict poor outcomes in patients with esophageal squamous cell carcinoma receiving combined modality therapy. Ann Surg Oncol 2014;21:2361-8. [Crossref] [PubMed]
- Ren Y, Cao B, Law S, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res 2005;11:6190-7. [Crossref] [PubMed]
- Hackel PO, Zwick E, Prenzel N, et al. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol 1999;11:184-9. [Crossref] [PubMed]
- Cai Z, Wang Q, Zhou Y, et al. Epidermal growth factor-induced epithelial-mesenchymal transition in human esophageal carcinoma cells--a model for the study of metastasis. Cancer Lett 2010;296:88-95. [Crossref] [PubMed]
- Ackland ML, Newgreen DF, Fridman M, et al. Epidermal growth factor-induced epithelio-mesenchymal transition in human breast carcinoma cells. Lab Invest 2003;83:435-48. [Crossref] [PubMed]
- Wang P, Ma M, Zhang S. EGF-induced urokinase plasminogen activator receptor promotes epithelial to mesenchymal transition in human gastric cancer cells. Oncol Rep 2017;38:2325-34. [Crossref] [PubMed]
- Rahbari NN, Schmidt T, Falk CS, et al. Expression and prognostic value of circulating angiogenic cytokines in pancreatic cancer. BMC Cancer 2011;11:286. [Crossref] [PubMed]
- Yang C, Bork U, Scholch S, et al. Postoperative course and prognostic value of circulating angiogenic cytokines after pancreatic cancer resection. Oncotarget 2017;8:72315-23. [Crossref] [PubMed]
- Hu Z, Xu R, Liu J, et al. GEP100 regulates epidermal growth factor-induced MDA-MB-231 breast cancer cell invasion through the activation of Arf6/ERK/uPAR signaling pathway. Exp Cell Res 2013;319:1932-41. [Crossref] [PubMed]
- Duriseti S, Goetz DH, Hostetter DR, et al. Antagonistic anti-urokinase plasminogen activator receptor (uPAR) antibodies significantly inhibit uPAR-mediated cellular signaling and migration. J Biol Chem 2010;285:26878-88. [Crossref] [PubMed]
- Andres SA, Edwards AB, Wittliff JL. Expression of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas and their clinical relevance. J Clin Lab Anal 2012;26:93-103. [Crossref] [PubMed]
- Dass K, Ahmad A, Azmi AS, et al. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev 2008;34:122-36. [Crossref] [PubMed]
- Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 2010;11:23-36. [Crossref] [PubMed]
- El-Kott AF, Khalil AM. El-Kenawy Ael-M. Immunohistochemical expressions of uPA and its receptor uPAR and their prognostic significant in urinary bladder carcinoma. Int Urol Nephrol 2004;36:417-23. [Crossref] [PubMed]
- Memarzadeh S, Kozak KR, Chang L, et al. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc Natl Acad Sci U S A 2002;99:10647-52. [Crossref] [PubMed]
- Gupta R, Chetty C, Bhoopathi P, et al. Downregulation of uPA/uPAR inhibits intermittent hypoxia-induced epithelial-mesenchymal transition (EMT) in DAOY and D283 medulloblastoma cells. Int J Oncol 2011;38:733-44. [PubMed]
- Gorantla B, Asuthkar S, Rao JS, et al. Suppression of the uPAR-uPA system retards angiogenesis, invasion, and in vivo tumor development in pancreatic cancer cells. Mol Cancer Res 2011;9:377-89. [Crossref] [PubMed]
- Baek MK, Kim MH, Jang HJ, et al. EGF stimulates uPAR expression and cell invasiveness through ERK, AP-1, and NF-kappaB signaling in human gastric carcinoma cells. Oncol Rep 2008;20:1569-75. [PubMed]


