Annual report of the esophageal cancer radiation group of the Department of Radiotherapy, Tianjin Medical University Cancer Institute & Hospital
Introduction
The Department of Radiotherapy of TJMUCH was founded in the 1950s by Prof. Jin Xianzhai, the late founder of oncology in China. It is one of the first and largest radiotherapy (RT) departments in China. After continuous efforts over half a century, it has developed into a well-known cancer radiation therapeutics center in China that integrates scientific research, teaching, and clinical treatments. At present, it is the national teaching and training base for RT physicians, the training base for Elekta, Inc. (Sweden) and the teaching and training base for postgraduates of Tianjin Medical University.
There are 5 wards and 310 beds in the radiation department. At present, 8 accelerators, 2 computed tomography (CT) machines for simulation, 1 magnetic resonance simulation machine, and 2 brachytherapy after-loading machines are in use. The department has approximately 200 staff members, including 50 physicians (10 of whom are chief physicians), 12 physicists, and approximately 50 technicians. In 2019, the area of the clinics and treatment rooms was 12,000 m2. There are 2 radiobiology laboratories covering an area of approximately 120 m2. The laboratories are equipped with two −80 °C refrigerators and three centrifuges with the capacity of simple processing of clinical specimens and basic molecular biology experiments.
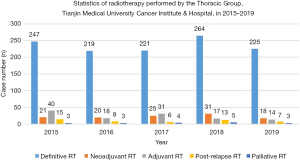
In 2019, the department treated approximately 6,000 new patients, including around 2,000 breast cancer patients, 1,500 lung cancer patients, 500 cervical cancer patients, and 500 patients with head and neck cancer. The esophageal cancer treatment group is part of the Department of Radiotherapy, TJMUCH, 5 physicians and 1 nutritionist are engaged in RT for esophageal cancer. Approximately 300 patients with esophageal cancer are admitted and treated each year (267 in 2019). Figure 1 shows the number and type of esophageal cancer cases admitted each year since 2015.
Esophageal cancer ranks seventh in incidence and sixth in cancer related death worldwide. Incidence rate is the highest in Eastern Asia with China in top 5 (1). Almost half of esophageal cancer occurs in China with squamous cell carcinoma as the main histology type (2). In China, the disease burden caused by esophageal cancer is heavy (3). Most patients are in the local progression stage of esophageal carcinoma when they seek medical consultation (4,5). According to CROSS and NEOCRTEC5010, neoadjuvant chemoradiotherapy (CRT) improved survival among patients with potentially resectable locally advanced esophageal cancer (6,7). Chen et al. demonstrated that cisplatin plus fluorouracil are the standard regimen in dCRT in patients with locally advanced esophageal squamous cell carcinoma (8). Definitive CRT and neoadjuvant CRT plus surgery have become standard therapies for locally advanced esophageal cancer patients. In the new era of immunotherapy, ATTRACTION-3 and KEYNOTE-181 have demonstrated promising antitumor effect of pembrolizumab (anti-PD-1-antibody) for progressed advanced esophageal cancer after first-line therapy (9). KEYNOTE-590 are ongoing to investigate anti-PD-1 pembrolizumab with or without chemotherapy as first-line treatment in patients with advanced esophageal or esophagogastric junction cancer (10). In the report, we will present our clinical work on the RT for esophageal cancer patients and our completed and ongoing clinical trials. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4064).
Methods
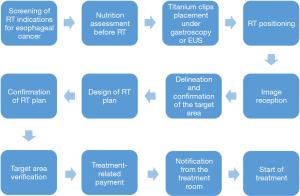
RT procedure for esophageal cancer patients: the esophageal cancer treatment group follows the RT procedure for the treatment of esophageal cancer patients (Figure 2).
For all patients diagnosed with esophageal cancer and will receive RT. we first screen patients for indications to determine the type of RT, including definitive CRT, preoperative neoadjuvant CRT, postoperative adjuvant RT, post-relapse RT and palliative RT. A pathology report must be confirmed before treatment. RT techniques include intensity modulated RT (IMRT) and three-dimensional conformal RT (3D-CRT).
Before RT, a well-trained nutritionist assesses the patient’s nutrition status with the NRS2002 Nutrition Screening Form and the Patient Generated Subjective Global Assessment (PG-SGA) Nutrition Assessment Form. Patients whose PG-SGA score are more than 4 will receive nutrition therapy, including nutrition education, oral nutrition supplements, nasogastric tube or nasointestinal tube, and percutaneous endoscopic gastrostomy (PEG) depending on different nutrition status before RT simulation. Nutrition therapy is performed by a nutrition therapy team consisting of 2 physicians, 1 nutritionist, 3 nurses, the patient and his or her family. After the start of RT, nutrition assessment is performed every week, and relevant data are recorded.
In addition, we use special metal titanium clips to mark margins of around 0.5 cm in the superior and inferior directions of esophageal cancer lesions before simulation. The procedure is performed by an endoscopy physician under the guidance of endoscopy or endoscopic ultrasound (EUS). In cases of severe obstruction that impedes the passage of endoscopic ultrasound, only the superior edge is marked. This marking procedure is omitted in patients aged 80 years and older.
RT simulation
For cervical and upper (1/3) thoracic esophageal cancers, the patient is fixed with a head, neck, and shoulder mask with arms on sides. For middle (1/3), lower (1/3), and gastroesophageal junction (GEJ) cancers, the patient is fixed with a chest mesh or negative pressure vacuum pad with arms up. Simulation is performed with routine contrast CT (slice thickness: 3–5 mm). Plain CT is performed in patients allergic to iodine or elderly patients, and patients with other complications. The scan ranges from the laryngeal node to the lower edge of the first lumbar vertebra. RT begins within 2 weeks of simulation.
RT techniques
RT techniques include IMRT and 3D-CRT. IMRT is the main treatment and includes volumetric modulated arc therapy (VMAT), and RAPID-ARC.
Target area and dosage of IMRT for esophageal cancer
The target area is defined by the physician and is then modified by the associate chief physician and finalized after group meeting with the chief physician and all team members. The target area covers the involved areas and is defined as follows: the gross tumor volume of the primary cancer (GTVp) is determined based on enhanced CT, positron emission tomography (PET)/CT, EUS, and upper gastrointestinal barium test; the upper and lower edges of the tumor are confirmed with titanium clips. The metastatic enlarged lymph node is delineated as regional lymph nodes (GTVnd) if the short diameter is greater than or equal to 5 mm or if the long diameter is greater than or equal to 10 mm based on CT, PET/CT, and EUS; the gross tumor volume (GTV) includes the primary tumor (GTVp) and involved GTVnd as identified on the planning scan. The clinical target volume includes the areas at risk for microscopic disease. Clinical target volume (CTV) was defined as the primary tumor plus a 3 cm expansion superiorly and inferiorly along the length of the esophagus and cardia, and a 0.6 cm radial expansion. The nodal clinical target volume should be defined by a 0.6 cm expansion from the nodal gross tumor volume. The planning target volume expansion (PTV) should be 0.5 cm beyond the CTV. The uncertainties arising from respiratory motion should also be taken into consideration. RT is delivered at 95% PTV: 60 Gy/2.0 Gy/30 f or concurrently at 95% of the planning gross target volume (PGTV): 60 Gy/2.0 Gy/30 f and 95% PTV: 54 Gy/1.8 Gy/30 f.
For cervical and upper thoracic esophageal cancer, the range for nodal irradiation includes the supraclavicular/lower neck lymphatic drainage area, the paraesophageal, paratracheal, subcarinal lymph nodes. For middle thoracic esophageal cancer, the range for nodal irradiation includes paraesophageal, paratracheal, subcarinal lymph nodes and lymph nodes along the left gastric artery (if the left gastric nodes are positive). For lower thoracic esophageal cancer, the range for nodal irradiation includes the paraesophageal nodes, subcarinal, paracardial lymph nodes; and lymph nodes along the left gastric artery. The dosage constrains for normal organs are as follows: 45 Gy for the spinal cord; 30 Gy with maximum of 40% of the total heart volume or 40 Gy with maximum of 30% of the total heart volume; 16 Gy for the maximum of the mean lung dose, 20 Gy with a maximum of 30% of the total lung volume, or 30 Gy with a maximum of 20% of the total lung volume.
Chemotherapy: the main regimen is weekly administration of docetaxel or paclitaxel combined with platinum-based drugs, including docetaxel 25 mg/m2/week and cisplatin 25 mg/m2/week or paclitaxel 40 mg/m2/week and cisplatin 25 mg/m2/week. An additional regimen is 3-week therapy with fluorouracil plus platinum (fluorouracil 500 mg/m2 on days 1–5, cisplatin 25 mg/m2 on days 1–3).
Quality control and treatment assurance
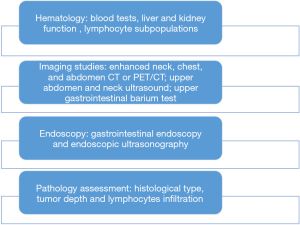
We follow the quality control principles of the treatment room and conduct morning inspections and dose rate measurements every day. During the treatment, two technicians are responsible for positioning and start the treatment only after electronic portal imaging device (EPID) or cone beam CT (CBCT) verification. During the course of RT (20 fractions), esophageal cancer patients undergo additional comprehensive exams and tests, similar to those performed at the initial visit (Figure 3) to further define the RT strategy (definitive RT/neo-adjuvant CRT) and adjust the target area.
Prospective clinical trials of RT for esophageal cancer
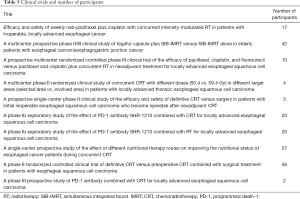
From January 2015 to December 2019, we participated in and were in charge of 10 clinical trials (Table 1) involving definitive RT plus immunotherapy for esophageal cancer, neoadjuvant CRT for esophageal cancer, and nutritional support during RT for esophageal cancer.
Full table
Results
In 2015–2019, 80% (1,176/1,464) of the esophageal cancer patients had locally advanced esophageal cancer and underwent definitive CRT. Only 7% patients (100/1,464) had resectable lesions, and they received preoperative CRT; postoperative adjuvant treatment included RT after radical resection of esophageal cancer, as well as endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) (5 cases in 2019). In addition, more patients with early esophageal cancer (T1bN0M0) are undergoing postoperative endoscopic procedures.
Definitive RT for esophageal cancer: between January 2015 and December 2019, 1,176 esophageal cancer patients received definitive CRT. The incidences of grade 2 and higher radiation esophagitis, radiation pneumonitis, and leukopenia were 19.4%, 3.6%, and 19.7%, respectively; the incidences of grade 3–4 radiation esophagitis, radiation pneumonitis, and leukopenia were 9.4%, 1.2%, and 5.4%, respectively; no grade 5 acute radiation adverse events were observed (Table 2). In 2015–2018, 44 patients (5%, 44/846) developed esophageal fistula; of these, 34 cases occurred after RT, and 10 cases occurred during RT.

Full table
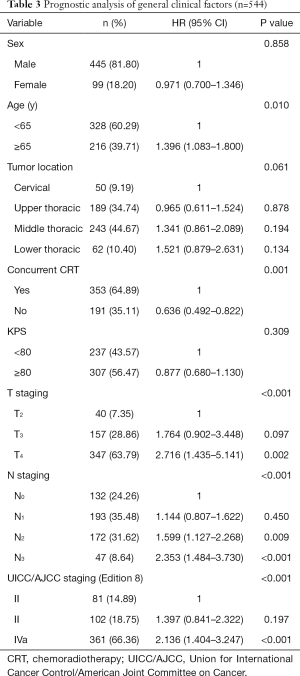
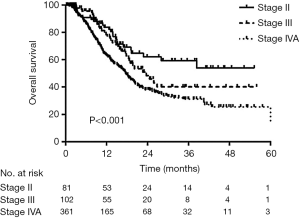
Outcomes of definitive RT for esophageal cancer: The long-term overall survival rate is not available for patients from 2015–2019. Thus, we used data from 544 patients with esophageal cancer who underwent definitive RT at TJMUCH between March 2010 and September 2016 instead. Ninety-nine patients were women, and 445 were men; 328 were under 65 years, and 216 were aged 65 years or older. Fifty patients had cervical esophageal cancer, 189 had upper thoracic esophageal cancer, 243 had middle thoracic esophageal cancer, and 62 had lower thoracic esophageal cancer. A total of 353 patients received concurrent CRT, and 191 patients did not. According to Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) staging (Edition 8), 81 were in stage II, 102 were in stage III, and 361 were in stage IVA. Table 3 shows the general characteristics of these patients. Follow-up lasted till July 15, 2018. The median follow-up time was 21.6 months, and 241 patients (44.30%) died. The median survival was 19.6 months; and the 1-, 3-, and 5-year overall survival rates were 69.4%, 37.2%, and 32.3%, respectively. The above survival rates were 83.8%, 59.3%, and 53.9% for patients in stage II, respectively; 78.4%, 40.1%, and 40.1% for patients in stage III, respectively; and 63.2%, 31.2%, and 25.7% for patients in stage IVA, respectively, (P<0.001) (Figure 4).
Full table
Neoadjuvant CRT for esophageal cancer
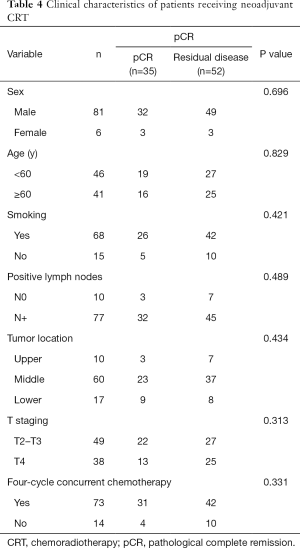
During the 5-year period (through December 2019), 100 patients received neoadjuvant CRT at our hospital. Of 87 patients who were followed for more than six months, 35 (40.2%) achieved pathological complete remission (pCR) after neoadjuvant CRT (40 Gy/20 f). Preliminary data show that sex, age, tumor location, stage, and course of chemotherapy had no significant effect on the pCR rate after neoadjuvant CRT (P>0.05) (Table 4).
Full table
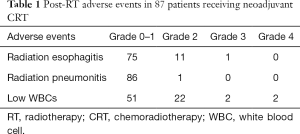
Table 1 shows the adverse reactions to neoadjuvant RT. Most grade 2 or above adverse reactions were radiation esophagitis (14%, 12/87) and low WBCs (27%, 24/87).
Prospective clinical trials of RT for esophageal cancer
From January 2015 to December 2019, we participated in and were in charge of 10 clinical trials (Table 5). Approximately 201 patients were enrolled, accounting for 13.6% (201/1,276) of esophageal cancer patients undergoing definitive or neoadjuvant CRT. The main research topics were definitive RT plus immunotherapy for esophageal cancer, comprising two phase Ib studies and one phase III prospective multicenter study (total case number: 42); neoadjuvant CRT combined with surgery for esophageal cancer, comprising three studies with 69 patients (69% of the patients undergoing neoadjuvant CRT); and a nutritional support study during definitive CRT for esophageal cancer (total case number: 27).
Full table
Discussion
This is the first annual report of the esophageal cancer treatment group of the Department of Radiotherapy, Tianjin Medical University Cancer Institute & Hospital. This report describes the clinical data of 1464 esophageal cancer patients who underwent RT at our hospital during the 5-year period between January 2015 and December 2019. Our hospital is one of the largest esophageal cancer RT centers in China and the world.
We have been standardizing RT procedures for esophageal cancer. RT is a complex procedure that encompasses the entire duration, from admission to the end of treatment, and requires multidisciplinary cooperation between internal medicine, RT, nutrition, surgery, and endoscopy. In this paper, we also present the details of the RT procedures. Our experience shows that pretreatment nutrition assessment (11-15), pretreatment nutrition therapy (16,17), pretreatment delineation of the target area, and efficacy evaluations throughout the course of treatment are all important.
Our standardized RT procedures ensure good treatment outcomes. The results show that the median survival was 19.6 months and the 1-, 3-, and 5-year overall survival rates were 69.4%, 37.2%, and 32.3%, respectively, which are superior to other reports from China and abroad (3-year survival: 20% to 30%) (18-22). We achieved superior results in resectable early esophageal cancer (T2, T3) patients relative to other studies (23). In addition, according to the Japan Clinical Oncology Group Study JCOG 0508 report this year, for patients in stage T1b receiving preventive CRT, the 3- and 5-year overall survival rates were as high as 91% and 89%, respectively (24,25). These data indicate that RT is an effective treatment for early esophageal cancer.
We are the first center in the world to investigate the effect of definitive RT/CRT combined with immunotherapy in patients with esophageal cancer. This study includes two phases. During phase one, patients who cannot tolerate or are unwilling to receive chemotherapy enrolled to the trial to test the safety of definitive RT combined with immunotherapy. Our phase two study was about definitive CRT combined with immunotherapy. Preliminary data shows that CRT plus immunotherapy is potentially effective, the incidence of grade 3–4 side effects in the CRT plus immunotherapy group is acceptable, indicating that it is a promising treatment option. At present, several multicenter clinical studies are underway in China and abroad, such as NCT 03278626 and NCT 03377400 (26,27).
In the past five years, 100 patients received preoperative CRT in our hospital, and 69 were enrolled in clinical trials. A total of 87 patients were included in analysis, and the results showed that 35 patients (40.2%) achieved pCR after neoadjuvant CRT (40 Gy/20 f), which is consistent with the results from the CROSS study (overall pCR rate: 29%; 49% of patients had squamous cell carcinoma) and the NEOCRTEC5010 study (pCR rate: 43.2%) (5,6). We evaluated tumor remission and tumor-infiltrating lymphocytes (TILs) during CRT as predictors of pathologic response and prognostic markers for patients with locally advanced ESCC treated with neoadjuvant CRT (neo-CRT) or definitive CRT (28). Furthermore, we conducted a prospective clinical study (NCT 02959385). In this study, esophageal cancer patients with CCR were randomized to undergo surgery or definitive CRT after neoadjuvant CRT. The preliminary survival analysis showed there was no significant difference in progression-free survival between these two groups. This study was chosen as oral presentation in the special session for esophageal cancer of the 2019 American Society for Radiation Oncology (ASTRO). High pCR and 3-year survival rates after neoadjuvant CRT challenge the necessity of surgery when patients achieving pCR (5-6,29). At present, the criteria for clinical complete response (cCR) varies from different centers. A more accurate criteria is needed to increase the consistency between cCR and pCR.
We attach great importance to nutritional support during the course of RT for esophageal cancer. Nutrition assessments are performed in more than 95% of esophageal cancer patients scheduled for RT and nutritional therapies are given to more than 80% of patients. Extensive nutritional assessment and intervention improve the tolerance of CRT and the treatment completion rate (>80%). Preliminary data show that nutrition therapy during CRT also reduces the incidence of esophagitis during and after RT.
Conclusions
Standardized treatment procedures, multidisciplinary cooperation, and the integration of clinical treatments and clinical trials are of great importance in esophageal cancer treatment and are the foundation for good treatment outcomes. We hope the outcomes of ongoing clinical trials with more patients enrolled in the near future could further improve treatment outcomes.
Acknowledgments
Funding: This study is supported by the Clinical Trial Supporting Foundation of Tianjin Medical University Cancer Institute & Hospital (Grant No. C1707), the National Nature Science Foundation of China, No. 81872462, and the National Nature Science Foundation of China, No. 81773300.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4064
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4064). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer 2016;7:232-7. [Crossref] [PubMed]
- Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol 2017;115:564-79. [Crossref] [PubMed]
- Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res 2018;30:1-12. [Crossref] [PubMed]
- Sun D, Cao M, Li H, et al. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin J Cancer Res 2020;32:129-39. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Chen Y, Ye J, Zhu Z, et al. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J Clin Oncol 2019;37:1695-703. [Crossref] [PubMed]
- Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506-17. [Crossref] [PubMed]
- Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol 2019;15:1057-66. [Crossref] [PubMed]
- Di Fiore F, Lecleire S, Pop D, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557-63. [Crossref] [PubMed]
- Wang J, Yu B, Ye Y, et al. Predictive Value of Nutritional Risk Screening 2002 and Prognostic Nutritional Index for Esophageal Cancer Patients Undergoing Definitive Chemoradiotherapy. Nutr Cancer 2018;70:879-85. [Crossref] [PubMed]
- Wang R, Cai H, Li Y, et al. Impact Exerted by Nutritional Risk Screening on Clinical Outcome of Patients with Esophageal Cancer. Biomed Res Int 2018;2018:7894084. [PubMed]
- Song T, Wan Q, Yu W, et al. Pretreatment nutritional risk scores and performance status are prognostic factors in esophageal cancer patients treated with definitive chemoradiotherapy. Oncotarget 2017;8:98974-84. [Crossref] [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Yu FJ, Shih HY, Wu CY, et al. Enteral nutrition and quality of life in patients undergoing chemoradiotherapy for esophageal carcinoma: a comparison of nasogastric tube, esophageal stent, and ostomy tube feeding. Gastrointest Endosc 2018;88:21-31.e4. [Crossref] [PubMed]
- Fietkau R, Lewitzki V, Kuhnt T, et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer 2013;119:3343-53. [Crossref] [PubMed]
- Minsky BD, Neuberg D, Kelsen DP, et al. Neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus: a preliminary analysis of the phase II intergroup trial 0122. J Clin Oncol 1996;14:149-55. [Crossref] [PubMed]
- al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997;15:277-84. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Minsky BD, Neuberg D, Kelsen DP, et al. Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90-12): Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 1999;43:517-23. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Kurokawa Y, Muto M, Minashi K, et al. A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol 2009;39:686-9. [Crossref] [PubMed]
- Minashi K, Nihei K, Mizusawa J, et al. Efficacy of Endoscopic Resection and Selective Chemoradiotherapy for Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology 2019;157:382-90.e3. [Crossref] [PubMed]
- Käsmann L, Eze C, Dantes M, et al. State of clinical research of radiotherapy/chemoradiotherapy and immune checkpoint inhibitor therapy combinations in solid tumours-a German radiation oncology survey. Eur J Cancer 2019;108:50-4. [Crossref] [PubMed]
- Liao XY, Liu CY, He JF, et al. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: A novel strategy. Oncol Lett 2019;18:5011-21. [PubMed]
- Qian D, Wang Y, Zhao G, et al. Tumor Remission and Tumor-Infiltrating Lymphocytes During Chemoradiation Therapy: Predictive and Prognostic Markers in Locally Advanced Esophageal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys 2019;105:319-28. [Crossref] [PubMed]
- Sasaki K, Uchikado Y, Omoto I, et al. Neoadjuvant chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-RT) for locally advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 2019;83:581-7. [Crossref] [PubMed]