
Ulinastatin alleviates cerebral ischemia-reperfusion injury in rats by activating the Nrf-2/HO-1 signaling pathway
Introduction
Stroke is an acute disease of the central nervous system that is mainly caused by cerebral ischemia (1). Annually, new cases of stroke total approximately 13.5 million worldwide, with 5.5 million deaths (2). Post-ischemic recanalization of potentially salvageable ischemic penumbra is the most effective therapy for stroke (3). However, even with timely restoration of blood flow (reperfusion), brain damage remains unavoidable; this is known as cerebral ischemia-reperfusion (I/R) injury. After reperfusion, structural and functional damage to brain tissue is often aggravated, and includes complex pathophysiological changes such as oxidative stress, excitotoxicity, inflammation, and apoptosis (4).
Ischemic damage in stroke develops from reactive oxygen species (ROS)-induced oxidative stress and glutamate-induced excitotoxicity, both of which cause rapid apoptotic cell death. Sustained inflammation also escalates damage progression. Currently, most therapeutic agents target ROS-induced oxidative stress, apoptosis, inflammation, and edema (5), which is understandable considering that glutamate is the most abundant excitatory neurotransmitter and thus may not be a suitable target for the prevention of ischemic damage. In contrast, ROS reduction can alleviate early apoptotic cell death, and the inhibition of inflammation can prevent further deterioration.
ROS generation and oxidative stress may be the driving forces of apoptosis, especially in cerebral ischemic stroke. Nuclear factor erythroid 2-related factor 2 (Nrf-2) is a central regulator of oxidative stress. Nrf-2 transcription initiation of antioxidant genes, especially heme oxygenase-1 (HO-1), is an important response to oxidative stress (6). Considering that brain tissue and neurons consume more oxygen than most organs, they more readily generate ROS under oxidative stress. Unsurprisingly, Nrf-2/HO-1 is an important therapeutic target of ischemic stroke, which is involved in the mechanism of action of many stroke therapeutics and pro-drugs, including natural products and synthesized chemicals (7,8).
Ulinastatin, a urinary trypsin inhibitor, is typically used in the clinical treatment of acute pancreatitis and as an auxiliary drug in the rescue of acute circulatory failure (9,10). Many studies have demonstrated the protective role of ulinastatin in I/R injury (including cerebral I/R injury in rats), which is related to oxidative stress, autophagy, and apoptosis (11,12). Nrf-2/HO-1 signaling is involved in the mechanism of action of ulinastatin in myocardial infarction and burns (13,14). In other diseases, Nrf-2 and HO-1 (alone or together) have been reported to reduce oxidative stress, apoptosis, and inflammation in response to ulinastatin administration (11,12,15). Still, further inquiry is required to determine the mechanism and extent of Nrf-2/HO-1 signaling in the protective effect of ulinastatin in cerebral I/R injury. In this study, cerebral I/R injury was modeled in rats to confirm the protective role of ulinastatin, and to identify the underlying mechanism(s). We observed that treatment with ulinastatin ameliorated cerebral I/R injury, and reduced inflammation and oxidative stress through activation of the Nrf-2/HO-1 signaling pathway.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5115).
Methods
Construction of cerebral I/R injury model
All animal studies were approved and supervised by the ethical committee of our hospital following the employed protocols (ACUC No: HDFY-LL-2016-19). All reported animals were experimented in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences. Pathogen-free, healthy male Sprague Dawley (SD) rats (two rats per cage. weight, 280±20 g) were purchased from and treated in Hebei Laboratory Animal Center. Rats were anesthetized with sodium pentobarbital (30 mg/kg) by intraperitoneal injection. Middle cerebral artery occlusion (MCAO) was used to model I/R injury, as previously described (16). Briefly, an incision was made in the middle of the rat’s neck, and the common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were separated. The proximal end of the CCA and the distal end of the ECA were ligated, and the distal end of the ICA was closed using a bulldog clamp. Next, an incision was made in the site 0.5 cm to the intersection of the ECA and ICA, and a nylon thread (0.3 mm in diameter) was inserted into the cut to a depth of 18.0±2.0 mm to induce cerebral ischemia via MCAO. After 2 hours of ischemia, the thread cord was removed to restore blood supply for 24 hours (reperfusion).
Animal grouping
The rats were randomly divided into 7 groups (8 rats per group, survived after treatment for 24 h) as follows: (I) the sham (control) group was operated with no ischemia; (II) the MCAO group was operated with MCAO; (III) the ulinastatin group was operated with MCAO and given ulinastatin at a dose of 300,000 U/kg (AmyJet Scientific Inc., Wuhan, China) (17); (IV) the ML-385 group was administered ML-385 (50 pmol/5 µL; AOBIOUS, MA, USA) and operated with MCAO (18); (V) the ulinastatin + ML-385 group was administered ulinastatin + ML-385 and then operated with MCAO; (VI) the Tert-butylhydroquinone (TBHQ) group was administered TBHQ (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) and operated with MCAO (18); (VII) the ulinastatin + TBHQ group was administered ulinastatin + TBHQ and then operated with MCAO. Ulinastatin was stored at 4 °C and dissolved in 0.9% saline upon use. Ulinastatin was injected intraperitoneally 15 min after MCAO (17). TBHQ and ML-385 were diluted in dimethyl sulfoxide (DMSO) before intracerebroventricular (i.c.v.) injection and administered 24 hours before MCAO (18). Upon completion of the above experiments, the blood and brain tissue of each rat was collected for analysis.
Evaluation of infarct volume by TTC staining
TTC staining was applied to estimate the infract volume according to previous report (19). Briefly, the collected brains were immediately placed in ice-cold saline for rinsing of the blood. Next, the brain slices (5–6 coronal slices of about 2 mm thick) were immediately stained with a 2% TTC solution at 37 °C for 30 min. The staining images were recorded by a digital camera and quantified analyzed by Image J software. The percent of total infract volume was calculated as the white area/total area ×100% of all brain slides.
Hematoxylin and eosin (HE) staining
Cerebral samples from the left ventricle were collected and fixed in a 4% buffered paraformaldehyde solution for 24 hours. Next, the tissues were dehydrated, embedded in paraffin, cut into 4-µm-thick sections for HE staining, and subsequently examined under light microscopy in six randomly selected areas.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
The 4-µm-thick sections of cerebral tissue were deparaffinized and rehydrated, and subsequently analyzed using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining to label the nuclei of apoptotic cells. Sections were first incubated in 3% H2O2 and then in the TUNEL reaction mixture (Roche Applied Science, Penzberg, Germany), according to the manufacturer’s instructions. The sections were rinsed and visualized using 3,3’-diaminobenzidine (DAB). Hematoxylin was used for counter-staining. The positive cells were observed under a light microscope (Olympus, Tokyo, Japan). The apoptosis index was calculated as the percentage of TUNEL-positive cells relative to the total number of cells.
Detection of oxidative stress index in brain tissue
The serum levels of ROS, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) were detected using commercial kits (Nanjing Jiancheng Biology Research Institute, Nanjing, China). The experiment was performed strictly according to the manufacturer’s instructions. Fluorescence was read with 500/530 nm (absorption/emission) for ROS. Optical absorption was assessed at 450 nm for SOD, 530 nm for MDA, and 410 nm for GSH using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of inflammatory cytokines in serum
Serum was separated from fresh blood to measure the levels of interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and interleukin 18 (IL-18). The levels of these inflammatory cytokines were determined using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blotting assay
Proteins were extracted from both ischemic and control brain cortices using Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Equal proteins were resolved on 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and then transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking, the membrane was incubated with the following primary antibodies (Cell Signaling, San Jose, CA, USA) at 4 °C overnight: Caspase-3 Antibody #9662, CD54/ICAM-1 (E3Q9N) XP® Rabbit mAb#67836, NRF-2 (D1Z9C) XP® Rabbit mAb#12721, HO-1 (E3F4S) Rabbit mAb #43966 and β-Actin (D6A8) Rabbit mAb #8457. Subsequently, anti-rabbit IgG, HRP-linked Antibody #7074 (Cell Signaling) was added for an incubation time of 1 hour. The proteins were detected using a ChemiDoc XRS imaging system, and image J software was employed to analyze the relative density of protein bands.
Statistical analyses
All data collected in this study were analyzed using GraphPad Prism software version 5.0. The data was expressed as the mean ± standard deviation (SD) of eight independent experiments. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test for intergroup comparisons. Differences were considered statistically significant at P<0.05.
Results
Ulinastatin ameliorates cerebral I/R injury and reduces apoptosis
Figure 1A shows representative images of HE and TUNEL staining. The sham group exhibited cells with a regularly organized arrangement and a well-outlined structure. However, the MCAO group exhibited irregular shapes and inflammatory infiltration. As expected, ulinastatin treatment ameliorated the damaged and irregularly arranged cerebral cells induced by the I/R injury. Moreover, TUNEL staining revealed that cells in the MCAO group were largely apoptotic, while ulinastatin treatment significantly reduced the rate of apoptosis in the ulinastatin group (Figure 1B, P<0.01). As shown in Figure 1C, TTC staining of the brain tissues showed that the infraction volume induced with MCAO was reduced with ulinastatin treatment, which was quantized in Figure 1D. Apoptosis and inflammation were briefly investigated, apoptosis-related protein caspase-3 and inflammation-related protein intercellular adhesion molecule 1 (ICAM-1) were highly expressed in the MCAO group brain tissue, while ulinastatin significantly reduced the expression of caspase-3 and ICAM-1 (Figure 1E, P<0.01). These results indicate that ulinastatin can ameliorate cerebral I/R injury and reduce apoptosis.
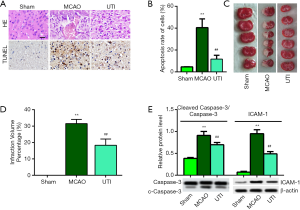
Ulinastatin inhibits oxidative stress and inflammatory cytokines induced by MCAO
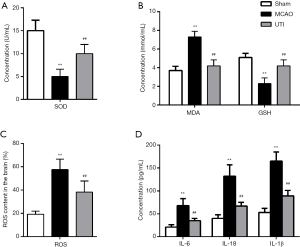
MCAOThe SOD activity (Figure 2A) and GSH (Figure 2B) content in the MCAO group were notably lower than those in the sham group, while the MDA (Figure 2B) and ROS (Figure 2C) levels in the MCAO group were significantly higher than that in the sham group (P<0.01). Importantly, the ulinastatin group showed elevated SOD activity and GSH content, and decreased MDA and ROS levels compared to the MCAO group (P<0.01), indicating that ulinastatin alleviates oxidative stress in cerebral I/R injury. Also, as shown in Figure 2D, the three inflammatory cytokines (IL-6, IL-1β, and IL-18) were elevated due to MCAO in the MCAO group compared to the sham group (P<0.01), while treatment with ulinastatin markedly decreased the levels of these cytokines in the MCAO group (P<0.01). This indicates that ulinastatin also mitigates inflammation in cerebral I/R injury.
Nrf-2/HO-1 is involved in Ulinastatin reduced I/R injury by MCAO
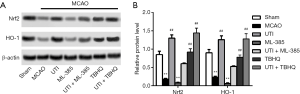
To assess whether Nrf-2 and HO-1 were involved in the mechanism of action of ulinastatin in ameliorating I/R injury, Nrf-2 activator TBHQ and inhibitor ML-385 were introduced into the experiments. As a result, relative protein expressions of Nrf-2 and HO-1 were decreased in the MCAO group, while ulinastatin and/or TBHQ significantly increased the expressions of Nrf-2 and HO-1 (Figure 3A,B, P<0.01). However, the inhibitory effect of ML-385 on the expression of Nrf-2 and HO-1 was not neutralized despite treatment with ulinastatin (P<0.01), indicating that activation of the Nrf-2/HO-1 signaling pathway could be necessary for ulinastatin to function.
Ulinastatin protects the brain from I/R injury through activation of Nrf-2/HO-1 pathway
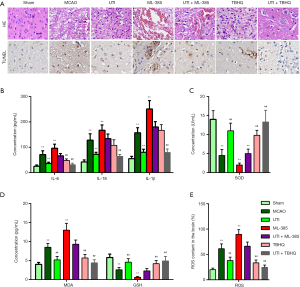
As shown by HE and TUNEL staining, TBHQ promoted while ML-385 counteracted the protective effects of ulinastatin on cerebral damage (Figure 4A). The inhibition of IL-6, IL-1β, and IL-18 levels by ulinastatin was nullified by ML-385 while enhanced with TBHQ (Figure 4B, P<0.01). Furthermore, the ulinastatin-induced GSH (Figure 4C) and SOD (Figure 4D) levels, and decreased MDA (Figure 4D) and ROS (Figure 4E) were enhanced with TBHQ while reverted by ML-385 (P<0.01). These results demonstrate that inactivation of the Nrf-2/HO-1 pathway could counteract the protective effects of ulinastatin on cerebral I/R injury in SD rats.
Discussion
Stroke is a leading cause of morbidity and mortality worldwide, and brings about various structural, functional, and molecular pathological changes in the brain. Treatment of stroke requires specialized targeting of several areas of its pathogenesis, particularly circulation, edema, and apoptosis of neuronal cells (20). However, after a stroke has occurred, brain damage is difficult to avoid, and cerebral I/R injury has presented a lasting challenge for therapy. Recent studies have shown that ulinastatin may be a promising medication for alleviating I/R injury (21), but unfortunately, the efficacy of ulinastatin for clinical use is not well established (22). Moving forward, it is necessary to further explore the mechanism(s) of action of ulinastatin so that effective novel therapeutics may be developed. Currently, studies have been directed toward several mechanisms of stroke, including the inhibition of apoptosis, permeability, and inflammation, or signaling pathways like p38 mitogen-activated protein kinase (MAPK), matrix metalloproteinase (MMP), and toll-like receptor-4 (TLR-4) (23,24). However, it is critical that greater attention be paid to the ability of ulinastatin to reduce oxidative stress. Considering that the brain is one of the most active oxygen consumers in the body, when cerebral I/R injury occurs, ROS are rapidly generated, inducing oxidative stress and excitotoxicity (5). Excitotoxicity is not a good therapeutic target considering that glutamate is the most abundant excitatory neurotransmitter. In other words, inhibiting the generation of ROS may be the most effective way to mitigate cerebral I/R injury in its early stages.
The effect of ulinastatin on ROS generation, as evidenced by reduced MDA and ROS, and increased SOD and/or GSH, has been observed in several studies, including those on myocardial infarction and transient cerebral ischemia in rats (14,21). The results gathered in this study are consistent with previous research in this area; we showed that MCAO induced an increase in MDA and ROS while reduced SOD and GSH, and that treatment with ulinastatin antagonized and reversed these changes. We also demonstrated that ulinastatin had an effect on the generation of ROS in cerebral I/R injuries, which was abolished by Nrf-2 inhibition (using ML-385).
Furthermore, ulinastatin has been shown to activate Nrf-2/HO-1, inhibiting myocardial infarction, oxidative stress, and inflammation in rats (13,14). Reduced ROS could be a result of ulinastatin activation of Nrf-2 in transient cerebral ischemic rats. In this study, we showed that up-regulation of HO-1, the inducible form of heme oxygenase, should be an important target of ulinastatin downstream of Nrf-2, as both Nrf-2 and HO-1 were significantly rescued by ulinastatin but inhibited by ML-385 (an Nrf-2 inhibitor) in MCAO rats.
Cell apoptosis and excitotoxicity have been shown to a play central role in cerebral I/R injury during oxidative stress burst (4). Upon burst of oxidative stress, ROS generation and mitochondrial dysfunction potently promote apoptosis. Targeting of apoptosis and related processes is critical for the treatment of cerebral I/R injury. According to recent studies, ulinastatin attenuated tissue injury and cell apoptosis in the brain, heart, kidneys, and intestines (25). Consistent with the aforementioned study, we observed that ulinastatin treatment significantly alleviated MCAO-induced I/R injury and apoptosis. Despite inhibiting apoptosis, however, ulinastatin did not restore cells, potentially due to evoked excitotoxicity or other unknown mechanisms. Thus, although targeting of ROS or Nrf-2/HO-1 may be effective, it is insufficient for the treatment of cerebral I/R injury unless rescue is expeditious.
Inflammation is the most critical post-ischemic degenerative factor, causing continued brain damage and leading to cerebral edema as well as the induction of increased numbers of inflammatory mediators (4). Reprogramming of inflammation signaling is an important therapeutic target. In recent studies, ulinastatin has been demonstrated to attenuate I/R injury (including cerebral ischemia) in multiple stressed organs by suppressing inflammation (23,26,27). ICAM-1 is a chemokine that recruits immune cells to evoke an inflammatory response; IL-6, IL-1β, and IL-18 were important inflammatory cytokines secreted under nucleotide oligomerization domain (NOD)-like receptor 3 (NLRP3)-enabled inflammation (28). In this study, ulinastatin treatment reduced the inflammatory response in SD rats, as evidenced by decreased ICAM-1 and inflammatory cytokines, including IL-6, IL-1β, and IL-18. Put simply, these results indicate that, through its inhibition of inflammation, ulinastatin may be beneficial in the post-ischemic phase of cerebral I/R injury.
Accumulating evidence indicates that Nrf-2 deficiency induces apoptosis, inflammation, and oxidative stress (29,30). In our study, we found that Nrf-2 and HO-1 were down-regulated in MCAO model rats, while treatment with ulinastatin rescued the reduced levels of Nrf-2 and HO-1. We utilized an Nrf-2 inhibitor (ML-385) to identify the role of ulinastatin on the Nrf-2/HO-1signaling pathway. ML-385 is a widely used inhibitor of Nrf-2 and is reported to effectively inhibit the Nrf-2/HO-1 signaling pathway (18). Consequently, while ML-385 was found to aggravate the cerebral I/R injury, ulinastatin counteracted the destructive role of ML-385. This indicates that ulinastatin alleviates cerebral I/R injuries by activating the Nrf-2/HO-1 signaling pathway.
The I/R injury protective role of ulinastatin was characterized in multiple organs including the brain, and its potential in promoting Nrf2 and HO-1 was also characterized (14,21). Thus, it seemed possible that ulinastatin would help improve I/R injury through reducing oxidative stress in clinic. However, there are limitations in this work. Especially, the possible relations between I/R injury, oxidative stress, Nrf2/HO-1 expression and ulinastatin remain largely unknown. Answer to these problems may benefit better treatment or drug discovery for I/R injury related brain diseases.
Conclusions
In conclusion, ulinastatin protected against cerebral I/R injury by inhibiting oxidative stress, apoptosis, and inflammation. Considering that ROS generation, oxidative stress, apoptosis, and inflammation were sequentially induced in cerebral I/R injury with interactions, the inhibition of these responses with ulinastatin treatment may stem from activation of the Nrf-2/HO-1 signaling pathway. These findings highlight Nrf-2/HO-1 signaling in the mechanism of action of ulinastatin as a promising potential therapeutic target for the treatment of cerebral I/R injury.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5115
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5115
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5115). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All protocols involving animal studies were approved by the Medical Ethics Committee of the Affiliated Hospital of Hebei, China (agreement 221/21.10.2018). All reported animals were experimented in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-the-care-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008;371:1612-23. [Crossref] [PubMed]
- Lindsay MP, Norrving B, Sacco RL, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2019. Int J Stroke 2019;14:806-17. [Crossref] [PubMed]
- Giebel B, Hermann DM. Identification of the right cell sources for the production of therapeutically active extracellular vesicles in ischemic stroke. Ann Transl Med 2019;7:188. [Crossref] [PubMed]
- Shahreyar M, Bob-Manuel T, Khouzam RN, et al. Trends, predictors and outcomes of ischemic stroke and intracranial hemorrhage in patients with a left ventricular assist device. Ann Transl Med 2018;6:5. [Crossref] [PubMed]
- Yang Q, Huang Q, Hu Z, et al. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front Neurosci 2019;13:1036. [Crossref] [PubMed]
- Li J, Zhao Y, Shi J, et al. Histone deacetylase 6 interference protects mice against experimental stroke-induced brain injury via activating Nrf2/HO-1 pathway. Anim Cells Syst (Seoul) 2019;23:192-9. [Crossref] [PubMed]
- Xie W, Zhou P, Sun Y, et al. Protective Effects and Target Network Analysis of Ginsenoside Rg1 in Cerebral Ischemia and Reperfusion Injury: A Comprehensive Overview of Experimental Studies. Cells 2018;7:270. [Crossref] [PubMed]
- Lv C, Maharjan S, Wang Q, et al. alpha-Lipoic Acid Promotes Neurological Recovery After Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage. Cell Physiol Biochem 2017;43:1273-87. [Crossref] [PubMed]
- Liu S, Xu J, Gao Y, et al. Multi-organ protection of ulinastatin in traumatic cardiac arrest model. World J Emerg Surg 2018;13:51. [Crossref] [PubMed]
- Li S, Yang W, Zhou L, et al. Vascular permeability and hemodynamic effects of ulinastatin on organs affected by shock during early burn injury. Am J Emerg Med 2019;37:249-53. [Crossref] [PubMed]
- Hu HX, Xu DH, Ju WN, et al. Neuroprotection of ulinastatin on transient cerebral ischemia via antioxidative mechanisms. J Biol Regul Homeost Agents 2018;32:283-8. [PubMed]
- Jiang XM, Hu JH, Wang LL, et al. Ulinastatin alleviates neurological deficiencies evoked by transient cerebral ischemia via improving autophagy, Nrf-2-ARE and apoptosis signals in hippocampus. Physiol Res 2018;67:637-46. [Crossref] [PubMed]
- He F, Song Y, Ying WJ, et al. Effects of Ulinastatin on myocardial oxidative stress and inflammation in severely burned rats. Eur Rev Med Pharmacol Sci 2018;22:5719-28. [PubMed]
- Wang S, Cheng ZY, Chen XJ, et al. Ulinastatin protects rats with myocardial infarction by activating Nrf2/NOS pathway. Eur Rev Med Pharmacol Sci 2018;22:8990-8. [PubMed]
- Song D, Song G, Niu Y, et al. Ulinastatin activates haem oxygenase 1 antioxidant pathway and attenuates allergic inflammation. Br J Pharmacol 2014;171:4399-412. [Crossref] [PubMed]
- Feng T, Han BH, Yang GL, et al. Neuroprotective Influence of miR-301a Inhibition in Experimental Cerebral Ischemia/Reperfusion Rat Models Through Targeting NDRG2. J Mol Neurosci 2019;68:144-52. [Crossref] [PubMed]
- Yano T, Anraku S, Nakayama R, et al. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology 2003;98:465-73. [Crossref] [PubMed]
- Zhang T, Xu S, Wu P, et al. Mitoquinone attenuates blood-brain barrier disruption through Nrf2/PHB2/OPA1 pathway after subarachnoid hemorrhage in rats. Exp Neurol 2019;317:1-9. [Crossref] [PubMed]
- Fang Y, Liu X, Zhao L, et al. RhGLP-1 (7-36) protects diabetic rats against cerebral ischemia-reperfusion injury via up-regulating expression of Nrf2/HO-1 and increasing the activities of SOD. Korean J Physiol Pharmacol 2017;21:475-85. [Crossref] [PubMed]
- Forouzanfar F, Shojapour M, Asgharzade S, et al. Causes and Consequences of MicroRNA Dysregulation Following Cerebral Ischemia-Reperfusion Injury. CNS Neurol Disord Drug Targets 2019;18:212-21. [Crossref] [PubMed]
- Hu HX, Zhu MQ, Sun YC, et al. Xuebijing enhances neuroprotective effects of ulinastatin on transient cerebral ischemia via Nrf2-ARE signal pathways in the hippocampus. J Biol Regul Homeost Agents 2018;32:1143-9. [PubMed]
- Lee B, Lee SY, Kim NY, et al. Effect of ulinastatin on postoperative renal function in patients undergoing robot-assisted laparoscopic partial nephrectomy: a randomized trial. Surg Endosc 2017;31:3728-36. [Crossref] [PubMed]
- Li X, Su L, Zhang X, et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res 2017;39:367-73. [Crossref] [PubMed]
- Li XF, Zhang XJ, Zhang C, et al. Ulinastatin protects brain against cerebral ischemia/reperfusion injury through inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins in mice. Exp Neurol 2018;302:68-74. [Crossref] [PubMed]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37:S186-202. [Crossref] [PubMed]
- Li ST, Dai Q, Zhang SX, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin 2018;39:1294-304. [Crossref] [PubMed]
- Song Y, Miao S, Li Y, et al. Ulinastatin attenuates liver injury and inflammation in a cecal ligation and puncture induced sepsis mouse model. J Cell Biochem 2019;120:417-24. [Crossref] [PubMed]
- Hu QH, Zhang X, Pan Y, et al. Allopurinol, quercetin and rutin ameliorate renal NLRP3 inflammasome activation and lipid accumulation in fructose-fed rats. Biochem Pharmacol 2012;84:113-25. [Crossref] [PubMed]
- Alfieri A, Srivastava S, Siow RCM, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med 2013;65:1012-22. [Crossref] [PubMed]
- Zhang W, Wei R, Zhang L, et al. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience 2017;366:95-104. [Crossref] [PubMed]
(English Language Editors: A. Kassem and J. Reynolds)


