
Antidepressant functions of Jie Yu Chu Fan capsule in promoting hippocampal nerve cell neurogenesis in a mouse model of chronic unpredictable mild stress
Introduction
Depression is a major public health challenge that has become the second leading cause of disability worldwide. It places a heavy economic and emotional burden on society. A meta-analysis of mental disorders found the population attributable risk for depression to be 11.2%, which was much higher than those of other types of mental disorders among suicides; in comparison, the population attributable risk for all causes was 12.7% (1). In a recent study, the overall incidence of depression among the elderly Chinese population was reported as 23.6% (2). Despite the effectiveness of conventional Western drugs in treating depression, approximately 40% of cases remain incurable (3). Furthermore, the initial side effects of current treatments in some patients with severe depression can include hysteria lasting for weeks or even months, during which time there is a high risk of self-harm or suicide (4). Moreover, Western medicines can have other serious side effects and withdrawal symptoms, which has prompted researchers to work to obtain a better understanding of the mechanisms of depression and to seek novel treatment drugs.
Chinese herbal medicine has a long history of safe use in the treatment of depression-like syndromes. Jie Yu Chu Fan (JYCF) capsule is a Chinese herbal medicine that is commonly prescribed for depression-like conditions. JYCF contains Gardenia jasminoides Ellis (ZZ), Magnolia officinalis (HP), Pinellia ternate Breit, and Forsythia suspensa, which are herbs commonly used to treat mental disorders, including depression (5-7). The clinical application of JYCF reflects its efficacy as an antidepressant; however, the underlying mechanism is still unclear.
Depression has been attributed to decreases in the levels of neurotransmitters, including 5-hydroxytryptamine (5-HT), norepinephrine (NE), and dopamine (DA) (8). Antidepressants enhance neuronal activities by increasing the levels of these neurotransmitters in the synaptic cleft (8,9). The regeneration of nerve cells in the hippocampus, which is regulated by brain-derived neurotrophic factor (BDNF), is crucial in the recovery of mice with chronic unpredictable mild stress (CUMS). Moreover, BDNF plays a pivotal role in structural plasticity, learning, and memory (10).
In the present study, we investigated the antidepressant-like activity of JYCF in comparison to a clinically prescribed antidepressant, fluoxetine, by establishing a mouse model of CUMS. Specifically, the concentrations of monoamine neurotransmitters and their metabolites as well as nerve cells at different stages and mRNA expression of BDNF in the hippocampi of the mice were measured by high-performance liquid chromatography (HPLC), immunofluorescence, and reverse transcription-polymerase chain reaction (RT-PCR), respectively.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5599).
Methods
Animals
The protocol of the animal experiments conducted in this study was approved by the Capital Medical University Animal Subjects Ethics Sub-Committee. The experiments were performed in compliance with guidelines of Capital Medical University for the care and use of animals. Eight-week-old male C57BL/6 mice were routinely housed in an animal facility at the Capital Medical University After 1 week of acclimatization, the animals were randomly assigned into five groups: the control group, the CUMS group, the CUMS + JYCF (1 g/kg) group, the CUMS + JYCF (5 g/kg) group, and the CUMS + fluoxetine group.
CUMS procedures
CUMS model was induced as previously reported (11). Briefly, the mice were subjected to the following mild stressors for 5 weeks in a random manner (one treatment per day) to ensure stress unpredictability: food and water deprivation for 24 hours; exposure to an empty bottle for 1 hour, 45° cage tilting for 7 hours, continuous illumination, damp bedding for 24 hours, swimming in cold water (temperature: 8 °C) for 6 minutes, physical restraint for 2 hours, and exposure to a foreign object for 4 hours. The depressive-like state of the mice was evaluated by behavioral tests, including the open field test, sucrose preference test, forced swim test, and tail suspension test.
After undergoing the behavioral tests, all of the animals were sacrificed under anesthesia with sodium pentobarbital (50 mg/kg). The brain of each mouse was immediately isolated, and the hippocampus was promptly collected and washed with saline. Half of the samples from each group were stored at −80 °C. For the other samples, intracardiac perfusion was performed with 0.9% saline, and the brain was stripped out entirely and fixed with paraformaldehyde.
Drug administration and sample collection
The CUMS mice were randomly divided into four treatment groups (6 mice/group) and orally fed with distilled water (the CUMS group), JYCF at 1 g/kg [the CUMS + JYCF (1 g/kg) group], JYCF at 5 g/kg [the CUMS + JYCF (5 g/kg) group], or fluoxetine at 20 mg/kg (the CUMS + fluoxetine group) for 5 weeks based on previous study (11). The dosages of JYCF and fluoxetine were selected based on their clinical dose and previous studies (11). Mice without stress stimulation were employed as control mice and orally given distilled water. Stress stimulations were conducted 2 hours after drug administration.
Body weight
The body weight of mice was measured before and after the CUMS procedure, as well as after the fluoxetine and JYCF treatments.
Open field test
The open field test was performed as previously described (11). The number of squares crossed by the animals and the frequency of standing on the hind limbs were respectively recorded over 3 minutes.
Sucrose preference test
As previously described (12), the mice were allowed to adapt to 1% sucrose solution before the test. For the test, the animals were presented with 2 bottles for 24 hours after 24 hours of water and food deprivation, 1 containing distilled water and the other containing 1% sucrose solution. The sucrose preference was calculated as the percentage of sucrose solution consumed versus the total water intake.
Forced swim test
The forced swim test was performed in a plexiglass cylinder (50 cm height, 20 cm diameter) containing water (35 cm depth). The mice were forced to swim for 15 minutes at room temperature (13). One day later, the animals were forced to swim for 6 minutes once more, and their immobility time in the last 4 minutes was rescored. The following actions were recognized as immobility: no struggling; upright floating posture; and occasional limb movement with the head above the water but without body movement (12).
Tail suspension test
The tail suspension test has been widely used for screening rodents in studies that focus on potential antidepressant drugs. Adhesive tape was placed approximately 1 cm away from the tip of the tail of the mice. Then, the mice were individually suspended 50 cm above the floor. The immobility time was recorded for the last 4 minutes of a 6-minute session, and the mice were considered as immobile when they hung passively and completely motionless.
HPLC-EC measurements of monoamine neurotransmitter and metabolic product levels
The contents of 5-hydroxytryptamine (5-HT), dopamine (DA), and norepinephrine (NE), as well as their respective metabolite 5-hydroxyindole acetic acid (5-HIAA), homovanillic acid (HVA), and 3,4-dihydroxyphenylacetic acid (DOPAC) in the hippocampi were determined by HPLC with electrochemical detection (HPLC-EC). Briefly, the mouse brain tissues were homogenized in 0.4 mL of pre-chilled internal labeling working solution at 4 °C. After centrifugation at 14,000 g for 15 minutes, the supernatant was collected to measure the concentration of neurotransmitters mentioned above by HPLC-EC column (ESA MD-150; 3.2 mm × 150 mm).
Immunofluorescence
Nerve cell regeneration in the mouse hippocampi was analyzed by immunofluorescence. Brain specimens were incubated with rabbit polyclonal antibody and subsequently with Alexa fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:100, Invitrogen) at room temperature, followed by 4’,6-diamidino-2-phenylindone (DAPI) staining of the nuclei. The images of the hippocampi were observed using 400× magnification and photographed using a fluorescence microscope (Olympus BX 51).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
Tissue sections were incubated in proteinase K, washed with phosphate-buffered saline (PBS), and covered in film. A reaction buffer containing TdT and UTP (v/v: 1:9) was added to the slices, which were then washed a second time. The nuclei were stained with DAPI. The samples were observed under a fluorescence microscope.
RNA extraction and RT-PCR
Total RNA was isolated from the hippocampi of the mice with TRIZOL reagent, following the manufacturer’s instructions (Invitrogen, USA). cDNA was produced with a reverse transcription kit (VAZYME) using 3 ug of RNA. The specific primers used for cDNA amplification were: GAPDH (forward: 5'-ATGGGTGTGAACCACGAGA-3', reverse: 5'-CAGGGATGATGTTCTGGGCA-3') and BDNF (forward: 5'-GCCTCCTCTACTCTTTCTG-3', reverse: 5'-GGATTACACTTGGTCTCGT-3'). The relative expression of the target gene was normalized by housekeeping gene GAPDH.
Statistical analysis
Data were presented as mean ± SD, and differences between groups were determined by one-way analysis of variance (ANOVA) with Dunnett’s post-hoc test. A P value of <0.05 was considered to be statistically significant.
Results
JYCF treatment altered CUMS-induced depression-like behaviors
To explore the effects of CUMS on animal behavior, the open field test, sucrose preference test, forced swim test, and tail suspension test were performed for all mice before and after the treatment. The weight of the mice was also recorded. As shown in Figure 1, in the CUMS model groups, there was significant weight loss after 5 weeks of CUMS exposure compared with the normal control group (P<0.01). JYCF treatment at both 1 and 5 g/kg, as well as fluoxetine, significantly reversed CUMS-induced weight loss (P<0.01) (Figure 1A).
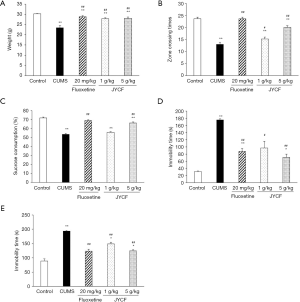
The results of the open field test showed that 5 weeks of exposure to CUMS significantly decreased the number of crossings compared with that of control group (P<0.01). Treatment with JYCF significantly increased the number of crossings (P<0.05) (Figure 1B).
After exposure to CUMS for 5 weeks, sucrose consumption in the CUMS mice was significantly reduced compared with that in the normal control group (P<0.01). Expectedly, fluoxetine induced a dramatic increase in sucrose consumption in the CUMS mice (P<0.05, Figure 1C). Similarly, JYCF at 5 g/kg resulted in a significant increase in sucrose preference in CUMS mice (P<0.01).
According to the results of the forced swim test, the immobility time of the mice was remarkably increased in the CUMS group compared with the control group (P<0.01). As with fluoxetine, JYCF at both 1 and 5 g/kg remarkably reduced the immobility time of the CUMS mice (P<0.05, Figure 1D). The results of the tail suspension test were similar to those of the forced swim test (Figure 1E).
Effects of JYCF treatment on neurotransmitters in the mouse hippocampus
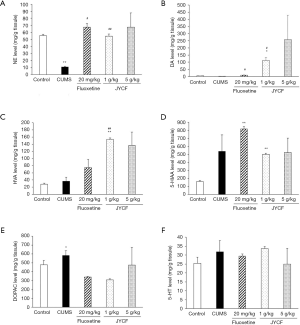
The contents of monoamine neurotransmitters and their metabolites in the mouse hippocampi were measured. After the administration of JYCF (1 g/kg) and fluoxetine (20 mg/kg), the contents of NE (Figure 2A) and DA (Figure 2B) remarkably increased compared with those in the CUMS group with no drug treatment (P<0.05). Treatment with JYCF at 1 g/kg significantly increased the level of HVA compared with that in CUMS group with no drug treatment (P<0.01, Figure 2C). No significant difference was found in the levels of 5-HIAA, DOPAC, or 5-HT after JYCF or fluoxetine treatment (Figure 2D,E,F).
JYCF stimulates nerve cell neurogenesis
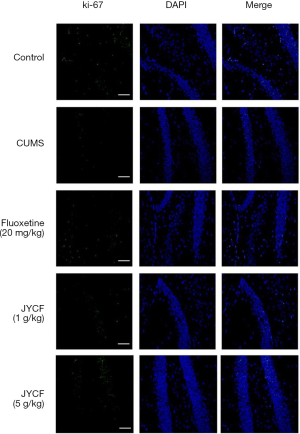
Ki-67 is a nuclear protein involved in cell proliferation. We compared its expression in the hippocampi of the mice in the different treatment groups (Figure 3). The results of immunofluorescence showed that after treatment with JYCF or fluoxetine, the expression of Ki-67 in each group was increased compared with the CUMS group with no drug treatment.
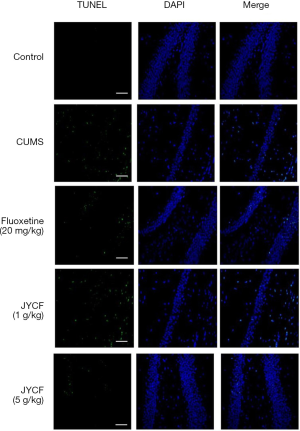
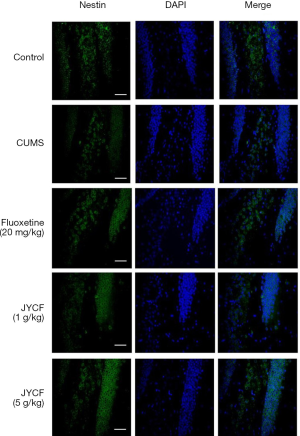
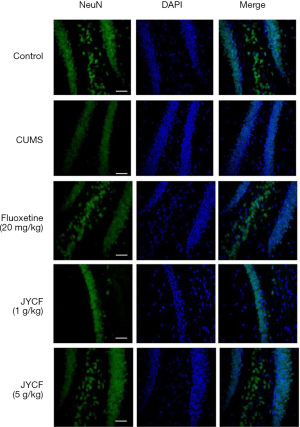
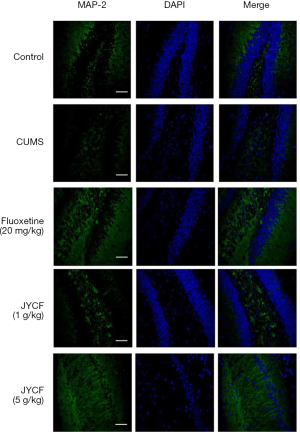
The results of TUNEL staining showed that following 35 days of treatment in the fluoxetine (20 mg/kg) and JYCF (5 g/kg) groups, the rate of apoptosis was decreased compared with that in the CUMS group with no drug treatment (Figure 4). To observe the nerve/progenitor cells in the hippocampi of the mice, nestin was used to perform immunofluorescence. The results revealed that nestin expression in the CUMS group with no drug treatment was decreased compared with that in the control group and that this phenomenon could be reverted with 35 days of treatment with fluoxetine or JYCF (Figure 5). The results of immunofluorescence showed a reduction in the levels of NeuN and MAP-2 in the CUMS group with no drug treatment compared with the control mice. After 5 weeks of treatment with fluoxetine or JYCF, the levels of NeuN and MAP-2 had increased to similar levels as those seen in the controls (Figures 6,7).
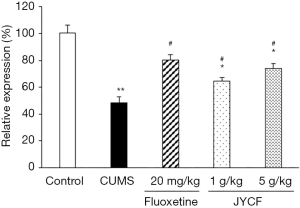
JYCF treatment increases BDNF mRNA expression in hippocampus of CUMS mice
Compared with the control group, BDNF mRNA expression was decreased in the CUMS group with no drug treatment, but could be restored by treatment with fluoxetine or JYCF (Figure 8).
Discussion
With a deepening understanding of the underlying molecular mechanisms of depression, numerous new therapies have been discovered for the treatment of this disorder. In this study, JYCF was characterized according to its therapeutic effects on depression. A CUMS model was used in this study, as described previously (11,14,15). Upon 5 weeks of unpredictable stimulation, the CUMS depression mouse model was successfully established, as indicated by the loss of body weight, zone crossing time in the open field test, sucrose preference, and increased immobility time in the CUMS mice, which were similar to those observed in previous studies (16,17).
The present study showed that after 35 days of repeated administration of JYCF or fluoxetine, the behavior of the CUMS mice recovered to some extent, which confirmed the antidepressant-like activity of JYCF. The CUMS mice exhibited reduced food intake and body weight, which are similar symptoms to those experienced by patients with major depressive disorder (MDD) (18). In our study, JYCF and fluoxetine significantly reversed the loss of body weight induced by CUMS in mice, which again agrees with the previous findings (6,10).
One of the core symptoms of MDD is anhedonia, which in the CUMS mice, was demonstrated by less sucrose consumption (14,19). Repeated administration of JYCF dramatically increased sucrose consumption and zone crossing times, which are the criteria for recognizing the antidepressant-like effects in the open field test (20). In the forced swim and tail suspension tests, immobility time was shortened compared with the CUMS group, which indicated that the depression had been alleviated (21). The results of the behavior test suggested that JYCF induced an antidepressant effect in the CUMS mouse model.
Monoamine transmitters play a critical role in stress response and in the modulation of depression (8,22). In the present study, CUMS mice had remarkable lower level of NE and DA in hippocampi comparing to that of normal control mice. Both fluoxetine and JYCF significantly increased NE and DA in CUMS mice. The increased levels of NE may directly enhance neurogenesis by promoting the proliferation of neural cells in the hippocampus (23). This action of NE on neurogenesis is mediated by β3-Ars, which contributes to the function of NE on neural stem cell activation and neurogenesis (24). DA may modulate depression-like and anxiety-like behaviors through the dopamine D1–D2 receptor heteromer (25,26). Some treatments for altered emotional states involve the rescue of abnormally low NE and DA levels. In our study, the level of NE and DA in the CUMS mice were dramatically reduced compared with the control mice. Treatment with JYCF or fluoxetine significantly reversed the changes in the levels of NE and DA in the CUMS mice without altering the level of 5-HT, suggesting that the antidepressant-like activity of JYCF might be mediated by regulating the levels NE and DA in the hippocampus.
Ki-67 is expressed in dividing cells and has been used as a marker of cell proliferation (27). Compared to CUMS group, fluoxetine and JYCF promoted the expression of Ki-67 in hippocampi, indicating the presence of new proliferative cells. Nestin-positive cells, as the proliferative progenitor neural cells (28), and MAP-2-positive cells, as the mature neurons, were enhanced after treatment with fluoxetine or JYCF. These findings were consistent with previous research on gradually improving depression (29). BDNF, a kind of the nerve growth factor, possesses the ability to support neuronal survival, differentiation, function and plasticity and has been observed to play a crucial role in certain neurobiological modifications that may otherwise lead to depression (30); CUMS exposure significantly decreased BDNF level in hippocampi, suggesting the involvement of BDNF in the pathology of depression, and treatment with fluoxetine and JYCF reversed CUMS-induced decrease in BDNF, which provided another possible mechanism of action for the antidepressant-like activity of JYC.
JYCF, a Chinese herbal medicine containing G. jasminoides Ellis (ZZ), Magnolia officinalis (HP), P. ternata Breit, and F. suspensa. Banxia Houpu decoction, including HP, P. ternata, Perilla frutescens, Poria cocos, and Zingiber officinale, has been widely used in the treatment of depression (6). F. suspensa has been reported to prevent the generation of reactive oxygen species that could be induced by chronic stress (31-34). In the present study, JYCF attenuated CUMS exposure-induced behavioral abnormalities and reversed the changes in NE, DA and its metabolite in hippocampi of CUMS mice without altering 5-HT level. Future study is needed to investigate in terms of the underlying mechanism of JYCF on the neurotransmitter system.
Conclusions
JYCF exhibited similar antidepressant-like effects to fluoxetine in the treatment of depression in CUMS mice. The essential neurotransmitters, NE and DA, as well as BDNF, a vital factor for improving nerve cell neurogenesis, were involved in mediating the anti-depressant effects of JYCF in CUMS mice.
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of China (No. 81703853).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5599
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5599
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5599). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol of the animal experiments conducted in this study was approved by the Capital Medical University Animal Subjects Ethics Sub-Committee. The experiments were performed in compliance with guidelines of Capital Medical University for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Walker ER, Mcgee RE, Druss BG. Mortality in Mental Disorders and Global Disease Burden Implications: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2015;72:334-41. [Crossref] [PubMed]
- Li D, Zhang J, Shao J, et al. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch Gerontol Geriatr 2014;58:1-9. [Crossref] [PubMed]
- Simon GE, Savarino J, Operskalski B, et al. Suicide Risk During Antidepressant Treatment. Am J Psychiat 2006;163:41-7. [Crossref] [PubMed]
- Zarate CA, Singh JB, Carlson PJ, et al. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry 2006;63:856-64. [Crossref] [PubMed]
- Sun Y, Feng F, Yu X. Pharmacokinetics of Geniposide in Zhi-Zi-Hou-Pu Decoction and in Different Combinations of its Constituent Herbs. Phytother Res 2012;26:67-72. [Crossref] [PubMed]
- Li JM, Kong LD, Wang YM, et al. Behavioral and biochemical studies on chronic mild stress models in rats treated with a Chinese traditional prescription Banxia-houpu decoction. Life Sci 2003;74:55. [Crossref] [PubMed]
- Ma Z, Ji W, Qu R, et al. Metabonomic Study on the Antidepressant-Like Effects of Banxia Houpu Decoction and Its Action Mechanism. Evid Based Complement Alternat Med. 2013;2013:213739.
- Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol 2004;28:435. [Crossref] [PubMed]
- Walker FR. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: Do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 2013;67:304-17. [Crossref] [PubMed]
- Geist PA, Dulka BN, Barnes A, et al. BNDF heterozygosity is associated with memory deficits and alterations in cortical and hippocampal EEG power. Behav Brain Res 2017;332:154-63. [Crossref] [PubMed]
- Ding L, Zhang X, Guo H, et al. The Functional Study of a Chinese Herbal Compounded Antidepressant Medicine-Jie Yu Chu Fan Capsule on Chronic Unpredictable Mild Stress Mouse Model. PLoS One 2015;10:e0133405. [Crossref] [PubMed]
- Xiang D, Wang H, Sun S, et al. GRP Receptor Regulates Depression Behavior via Interaction With 5-HT2a Receptor. Front Psychiatry 2020;10:1020. [Crossref] [PubMed]
- Yankelevitch-Yahav R, Franko M, Huly A, et al. The Forced Swim Test as a Model of Depressive-like Behavior. J Vis Exp 2015;97:52587. [PubMed]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005;52:90-110. [Crossref] [PubMed]
- Brenes Sáenz JC, Villagra OR, Barnes A, et al. Factor analysis of Forced Swimming test, Sucrose Preference test, and Open Field test on enriched, social, and isolated reared rats. Behav Brain Res 2006;169:57-65. [Crossref] [PubMed]
- Bhatt S, Mahesh R, Jindal A, et al. Protective effects of a novel 5-HT3 receptor antagonist, N-n-butyl-3-methoxy quinoxaline-2-carboxamide (6o) against chronic unpredictable mild stress-induced behavioral changes and biochemical alterations. Pharmacol Biochem Behav 2014;122:234-9. [Crossref] [PubMed]
- Qiu J, Hu S, Zhang C, et al. The effect of Chaihu-Shugan-San and its components on the expression of ERK5 in the hippocampus of depressed rats. J Ethnopharmacol 2014;152:320-6. [Crossref] [PubMed]
- Brunner E, Tohen M, Osuntokun O, et al. Efficacy and Safety of Olanzapine/Fluoxetine Combination vs Fluoxetine Monotherapy Following Successful Combination Therapy of Treatment-Resistant Major Depressive Disorder. Neuropsychopharmacology 2014;39:2549-59. [Crossref] [PubMed]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 1997;134:319-29. [Crossref] [PubMed]
- Wang X, Xiu Z, Du Y, et al. Brazilian Treatment Produces Antidepressant- and Anxiolytic-Like Effects in Mice. Biol Pharm Bull 2019;42:1268-74. [Crossref] [PubMed]
- Han YX, Chen T, Gao XR, et al. BDNF-Related Imbalance of Copine 6 and Synaptic Plasticity Markers Couples With Depression-Like Behavior and Immune Activation in CUMS Rats. Front Neurosci 2018;12:731. [Crossref] [PubMed]
- Hou C, Jia F, Liu Y, et al. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res 2006;1095:154-8. [Crossref] [PubMed]
- Masuda T, Nakagawa S, Boku S, et al. Noradrenaline increases neural precursor cells derived from adult rat dentate gyrus through beta2 receptor. Prog Neuropsychopharmacol Biol Psychiatry 2012;36:44-51. [Crossref] [PubMed]
- Jhaveri DJ, Mackay EW, Hamlin AS, et al. Norepinephrine directly activates adult hippocampal precursors via β3-adrenergic receptors. J Neurosci 2010;30:2795. [Crossref] [PubMed]
- Shen M, Perreault M, Bambico F, R, et al. Rapid anti-depressant and anxiolytic actions following dopamine D1-D2 receptor heteromer inactivation. Eur Neuropsychopharmacol 2015;25:2437-48. [Crossref] [PubMed]
- Hasbi A, Perreault L, Shen M, et al. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo: effective selective antagonism. FASEB J 2014;28:4806-20. [Crossref] [PubMed]
- Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol 2000;182:311-22. [Crossref] [PubMed]
- Cicvaric A, Sachernegg M, Stojanovic T, et al. Podoplanin gene disruption in mice promotes in vivo neural progenitor cells proliferation, selectively impairs dentate gyrus synaptic depression and induces anxiety-like behaviors. Front Cell Neurosci 2020;13:561. [Crossref] [PubMed]
- Hu Z, Du X. Yang Yet al. Progesterone and fluoxetine treatments of postpartum depressive-like behavior in rat model. Cell Biol Int 2019;43:539-52. [Crossref] [PubMed]
- Peng S, Li W, Lv L, et al. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov Med 2018;26:127-36. [PubMed]
- Lucca G, Comim M, Valvassori S, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 2009;54:358-62. [Crossref] [PubMed]
- Iguchi Y, Kosugi S, Nishikawa H, et al. Repeated Exposure of Adult Rats to Transient Oxidative Stress Induces Various Long-Lasting Alterations in Cognitive and Behavioral Functions. PLOS ONE 2014;9:e114024. [Crossref] [PubMed]
- Huang C, Lin Y, Su H, et al. Forsythiaside Protects Against Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis in PC12 Cell. Neurochem Res 2015;40:27-35. [Crossref] [PubMed]
- Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008;11:851-76. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


