
Comparison of a new anterior segment optical coherence tomography and Oculus Pentacam for measurement of anterior chamber depth and corneal thickness
Introduction
Accurate biometric measurements of the anterior ocular segment are of great importance for both diagnostic and therapeutic purposes. Measurement of the anterior chamber depth (ACD) is important when monitoring the changes of the anterior segment during accommodation (1), screening of primary angle-closure glaucoma (2), and performing the optical power calculation of phakic and pseudophakic intraocular lenses (IOLs) (3-5). Measurement of central corneal thickness (CCT) is essential for planning refractive surgery procedures (6,7), monitoring corneal conditions (8) and accurate measurement of intraocular pressure (9,10). To add, peripheral corneal thickness has also been proposed to be a useful parameter for the clinical identification of corneal disease with corneal thinning such as keratoconus (11,12).
Nowadays, increased attention has been paid to ACD (13-15) and regional corneal thickness measurements (12,16) from different devices. The Pentacam (Oculus Inc., Wetzlar, Germany) is a rotating Scheimpflug system which makes the noninvasive assessment of the anterior chamber structures accessible (16). Many studies have proved it as a noninvasive, repeatable, accurate, and reliable method for the measurement of corneal thickness and ACD (17-19).
Lately, new instruments have been utilized to measure corneal thickness and ACD. Anterior segment optical coherence tomography (AS-OCT) can be used to examine the cornea or anterior segment and can provide qualitative and quantitative information to assess the corner and anterior chamber (20-22). The CASIA2 AS-OCT (Tomey, Nagoya, Japan) is a novel swept-source optical coherence tomography (OCT) with a faster scan speed of 50,000 A-scans per second, a much wider and deeper imaging, and a higher-resolution imaging of 800 A/B-scans, a more advanced development from the prior generation CASIA SS-1000.
Several previous studies have compared ocular parameters such as ACD, angle-to-angle (ATA) distance, white-to-white (WTW) distance and pupil size using CASIA SS-1000 and various instruments other than Pentacam (23-27). One study has compared the measurement of aqueous depth (AQD) performed by CASIA2 and Pentacam (28). However, there is not yet a study that compares the CASIA2 AS-OCT device with the Pentacam in measuring central and peripheral corneal thickness or ACD. The purpose of this study is to compare central and regional corneal thickness values and ACD values obtained by CASIA2 and those obtained by Pentacam, and to evaluate the reliability and validity of the CASIA2 AS-OCT.
Methods
This prospective cross-sectional study was performed from May 2018 to July 2018 at the Zhongshan Ophthalmic Center at Sun Yat-sen University in Guangzhou, China, with forty-nine healthy subjects recruited. All subjects were informed of the experimental purpose and procedures in detail before each examination was performed. The study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional board of Zhongshan Ophthalmic Centre of Sun Yat-sen University (IRB-ZOC-SYSU) and informed consent was taken from all the participants. All records and information of participants were anonymized and de-identified before being analyzed.
Subjects and measurements
The inclusion criteria were as follows: (I) participants older than 18 years of age and (II) no history of ocular disease such as glaucoma or cornea disease, as this would affect ACD and corneal thickness. Participants with any ocular surgery or ocular trauma were also excluded. All eligible subjects in our study were randomly selected through recruitment according to the above inclusion criteria.
The exclusive criteria were as follows: (I) spherical equivalent power greater than −6.00 D; (II) wearing contact lenses within the past 2 weeks of recruitment date; and (III) severe cataract or with apparent ocular diseases (such as corneal scarring, keratoconus, active ocular surface disease, pterygium, or retinal detachment). Subjects that displayed low levels of cooperation or study compliance were also excluded.
The right eye of each subject was scanned with both Pentacam (Oculus Inc., Wetzlar, Germany) and CASIA2 (Tomey Corporation, Nagoya, Japan) without dilation after 5 minutes of dark adaptation. Each examination was performed by the same experienced operator. Before each measurement, the operator would explain to patients the procedure for each examination. To ensure all images were obtained without lid artefacts, the operator held the subjects’ eyelid gently while avoiding pressing the eyeball. To prevent illumination—dependent or time—differences in ocular anatomy, the sequence of the devices was selected randomly.
The Pentacam system uses a rotating Scheimpflug camera (360 degrees) and a monochromatic slit-light source (blue light–emitting diode at 475 nm) which rotate together around the optical axis of the eye to calculate a 3-dimensional (3D) model. It can capture 25 slit-images of the anterior chamber per measurement in 2 seconds automatically.
For CASIA2, all subjects were measured under non-mydriatic conditions after 5 minutes of adaptation under dark room conditions. The CASIA2 is a novel swept-source OCT specifically designed for imaging the anterior segment. It uses 1,310 nm swept-source laser wavelength at a frequency of 0.3 seconds, producing 128 cross-sectional images performed evenly spaced 1.4 degrees apart.
The parameters involved in this study were all automatically calculated by the two devices, and were documented by two independent investigators after examination. Parameters collected for comparison included CCT and ACD, and the two devices both had peripheral corneal thickness measurements obtained at the 6mm radius in the superior, inferior, nasal and temporal regions.
Statistical analysis
All statistical analyses were performed by Stata 12.0 (Stata Corp., College Station, TX, USA). The Kolmogorov-Smirnov test was used to check normality of all the parameter values, and mean ± standard error (SD) was used for statistical description. The coefficient of repeatability (COR =1.96-fold SD), the relative COR (rCOR = COR/average measurement) and the limits of agreement (LOA = mean ± COR) were calculated to assess the repeatability of CASIA2 and the agreement of CASIA2 and Pentacam. The Bland-Altman plot was used to assess the agreement between parameters from the two devices. P values <0.05 were considered statistically significant.
Results
In total, data from forty-nine eyes of forty-nine healthy adults were analyzed. The mean ± age of the subjects was 24.78±4.36 (range, 18–36) years old. The 49 subjects consisted of 28 males and 21 females.
The mean difference, COR, relative COR, lower and upper LOA and P values of the two measurements obtained by CASIA2 are shown in Table 1. For the two examinations of corneal thickness with CASIA2, CCT has the lowest COR with 18.58 µm and the inferior corneal thickness (ICT) has the highest COR with 28.32 µm. For the repeatability measurement of ACD by CASIA2, the COR was 0.31 mm. However, the P value suggests there is no statistically significant difference between the two measurements both for corneal thickness and for ACD by CASIA2 (P>0.05).
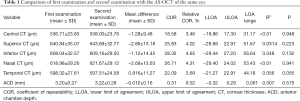
Full table
For the agreement of corneal thickness and ACD measured by Pentacam and CASIA2, the mean difference of CCT, superior corneal thickness (SCT), ICT, nasal corneal thickness (NCT), temporal corneal thickness (TCT) and ACD were 9.64, 25.22, 14.66, 29.86, 22.04 µm and −0.075 mm, respectively. COR was 13.10, 51.57, 48.06, 56.21, 47.69 µm and 0.294 mm, respectively. The mean difference and COR of peripheral corneal thickness was significantly higher than that of CCT (Table 2).
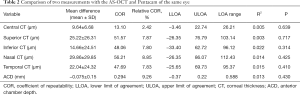
Full table
The Bland-Altman analysis of corneal thickness obtained by Pentacam and CASIA2 is shown in Figures 1,2. The Bland-Altman analysis of CCT for the examination with Pentacam and CASIA2 shows a mean difference of 9.64 µm, COR of 13.10 µm, relative COR of 2.42% with P=0.639 (Figure 1). There was also no statistically significant difference for measurements of peripheral corneal thickness between Pentacam and CASIA2 (P=0.717 for SCT, P=0.314 for ICT, P=0.425 for NCT, P=0.410 for TCT).
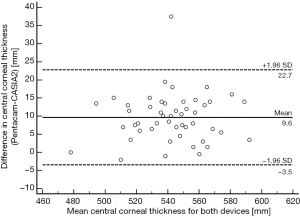
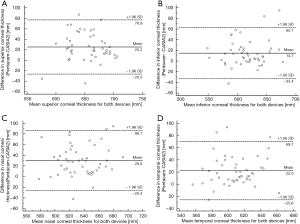
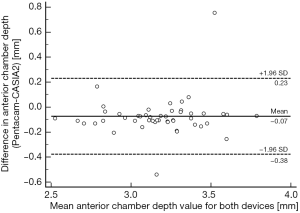
Comparing the two measurements of the Pentacam and CASIA2, the mean difference, COR, and LOA range of ACD was −0.075, 0.294 and 0.588 mm, respectively, which were lower than that of corneal thickness. There was no significant difference between the two measurements of the two devices with P=0.4304 (Figure 3).
Discussion
Central and peripheral corneal thickness evaluation is important for planning keratorefractive surgical procedures (6,7), monitoring corneal conditions (8), diagnosing keratoconus (11,12) and assessing intraocular pressure accurately (9,10). ACD, another important ocular parameter, has become an essential factor for IOL power calculations in patients about to undergo cataract surgery (3,4). In our study, we assessed the repeatability of the automatic corneal thickness and ACD measurements provided by CASIA2 AS-OCT and compared systematic difference of evaluation obtained with CASIA2 AS-OCT and Pentacam Scheimpflug imaging system for normal eyes. CASIA2 demonstrated excellent intradevice reproducibility for both central and peripheral corneal thickness and ACD. In addition, there were no statistically significant differences between Pentacam and CASIA2 in these parameters.
We reported the average CCT was 537.36±23.33 µm measured by CASIA2, which was similar to the results published by Simon and Katarzyna (29,30). In the current study, our results show that the average ACD is 3.21±0.28 mm measured by CASIA2, which are similar to the results reported by Xu et al., who reported a mean ACD value of 3.13±0.29 mm in healthy subjects (31). For the repeatability of CASIA2, our results show that there is no statistically significant difference between the two examinations in healthy subjects not only for corneal thickness in different regions but also for ACD (all P>0.05). Moreover, small relative COR values of CCT, SCT, ICT, NCT, TCT and ACD (3.46%, 4.02%, 4.65%, 4.31%, 3.69% and 9.52%, respectively) also suggested high-repeatability for regional corneal thickness and ACD measurements with CASIA2 AS-OCT in normal eyes, indicating that the CASIA2 could provide good reproducible measurements for the corneal thickness in different regions and ACD.
Many studies have evaluated the accuracy of the Pentacam measurement of ACD and corneal thickness parameters (32,33). So far, there have been few studies comparing corneal thickness and ACD measured by Pentacam and Swept-Source OCT. Fukuda et al. reported that there was no statistically significant difference in the CCT and AQD between the CASIA2 and CASIA SS-1000 (28). The results reported by Krysik et al. show the average CCT evaluated by Pentacam is significantly higher than that obtained by CASIA SS-1000 (30). In our study, Pentacam pachymetry measurements also show slightly higher CCT values when compared with CASIA2 measurements. However, there was no statistical significance in the differences of corneal thickness in different regions and ACD measured by the two devices (P>0.05). The differences in values of measured parameters may be possible due to the systematic difference. Small relative COR values of CCT, SCT, ICT, NCT, TCT and ACD (2.42%, 7.87%, 7.80%, 8.85%, 7.83% and 9.26%, respectively) also suggest that the Pentacam and the CASIA2 had excellent agreement. Moreover, the Bland-Altman plots of corneal thickness and ACD showed a good correlation between the two devices.
One advantage of the CASIA2 AS-OCT is that it was specifically designed for imaging the anterior segment. With a scan speed of 50,000 A-scans per second, and a frame size of 800 A-scans, it takes only 0.016 s to capture a single cross-sectional image (34). The shortened scanning time can reduce the effects of motion artifacts resulting from involuntary ocular movement and patient stress, making it easier for patients to tolerate and cooperate with examinations.
Another advantage of this AS-OCT scanner is that it uses a 1,310 nm infrared light source, which can minimize the effects of the measurement light on pupil movement and miosis (34). Moreover, this kind of light theoretically achieves better penetration of light into the opaque tissues than the visible light (475 nm) used in the Pentacam (35,36). Because some corneal opacities or scars may scatter the visible light in the Pentacam, this can make it difficult to digitize the corneal surfaces precisely. Using the AS-OCT, we can accurately measure corneal parameters of patients with a history of corneal disease or corneal surgery.
Our study shows that the AS-OCT is reliable, valuable, and accurate in terms of corneal thickness and ACD measurements, meaning that the AS-OCT is an option to be considered in the diagnosis of corneal disease and planning of refractive surgery. However, our findings are limited to healthy Chinese people, as the study only includes Chinese people without a history of previous ocular surgery or remarkable ocular and systemic diseases. Therefore, more multi-center clinical trials are still needed to verify the validity and reliability of AS-OCT in terms of corneal thickness and ACD measurements in different populations in the future.
Conclusions
In conclusion, CASIA2 offered excellent repeatability for corneal thickness and ACD measurements in healthy subjects and good agreement with Pentacam. CASIA 2 can be used as an alternative to Pentacam for measuring corneal thickness and ACD when monitoring corneal conditions, measuring intraocular pressure or planning ocular surgery. We believe there is value in the reliability and clinical convenience of the automatic corneal thickness and ACD measurements with CASIA2 AS-OCT, and its faster scanning speed, higher resolution, and deeper and wider scanning range may be useful in the diagnosis of various types of corneal diseases. However, subtle differences between the CASIA2 and Pentacam should also be kept in mind for certain specific clinical or research purposes.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (81873673).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-187
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-187). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and was approved by institutional board of Zhongshan Ophthalmic Centre of Sun Yat-sen University (IRB-ZOC-SYSU) and informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Langenbucher A, Huber S, Nguyen NX, et al. Measurement of accommodation after implantation of an accommodating posterior chamber intraocular lens. J Cataract Refract Surg 2003;29:677-85. [Crossref] [PubMed]
- Devereux JG, Foster PJ, Baasanhu J, et al. Anterior chamber depth measurement as a screening tool for primary angle-closure glaucoma in an East Asian population. Arch Ophthalmol 2000;118:257-63. [Crossref] [PubMed]
- Olsen T, Corydon L, Gimbel H. Intraocular lens power calculation with an improved anterior chamber depth prediction algorithm. J Cataract Refract Surg 1995;21:313-9. [Crossref] [PubMed]
- Holladay JT. Standardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculations. J Cataract Refract Surg 1997;23:1356-70. [Crossref] [PubMed]
- Lee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation. Curr Opin Ophthalmol 2008;19:13-7. [Crossref] [PubMed]
- Zhao MH, Zou J, Wang WQ, et al. Comparison of central corneal thickness as measured by non-contact specular microscopy and ultrasound pachymetry before and post LASIK. Clin Exp Ophthalmol 2007;35:818-823. [Crossref] [PubMed]
- Ciolino JB, Khachikian SS, Belin MW. Comparison of corneal thickness measurements by ultrasound and scheimpflug photography in eyes that have undergone laser in situ keratomileusis. Am J Ophthalmol 2008;145:75-80. [Crossref] [PubMed]
- Muscat S, McKay N, Parks S, et al. Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Invest Ophthalmol Vis Sci 2002;43:1791-5. [PubMed]
- Bechmann M, Thiel MJ, Roesen B, et al. Central corneal thickness determined with optical coherence tomography in various types of glaucoma. Br J Ophthalmol 2000;84:1233-7. [Crossref] [PubMed]
- Sullivan-Mee M, Halverson KD, Saxon GB, et al. The relationship between central corneal thickness-adjusted intraocular pressure and glaucomatous visual-field loss. Optometry 2005;76:228-38. [Crossref] [PubMed]
- Pflugfelder SC, Liu Z, Feuer W, et al. Corneal thickness indices discriminate between keratoconus and contact lens-induced corneal thinning. Ophthalmology 2002;109:2336-41. [Crossref] [PubMed]
- Randleman JB, Lynn MJ, Perez-Straziota CE, et al. Comparison of central and peripheral corneal thickness measurements with scanning-slit, Scheimpflug and Fourier-domain ocular coherence tomography. Br J Ophthalmol 2015;99:1176-81. [Crossref] [PubMed]
- Lavanya R, Teo L, Friedman DS, et al. Comparison of anterior chamber depth measurements using the IOLMaster, scanning peripheral anterior chamber depth analyser, and anterior segment optical coherence tomography. Br J Ophthalmol 2007;91:1023-6. [Crossref] [PubMed]
- Shajari M, Cremonese C, Petermann K, et al. Comparison of Axial Length, Corneal Curvature, and Anterior Chamber Depth Measurements of 2 Recently Introduced Devices to a Known Biometer. Am J Ophthalmol 2017;178:58-64. [Crossref] [PubMed]
- Wang Q, Ding X, Savini G, et al. Anterior chamber depth measurements using Scheimpflug imaging and optical coherence tomography: repeatability, reproducibility, and agreement. J Cataract Refract Surg 2015;41:178-85. [Crossref] [PubMed]
- Ceylan OM, Turk A, Erdurman C, et al. Comparison of Oculus Pentacam and Stratus optical coherence tomography for measurement of central corneal thickness. Cornea 2011;30:670-4. [Crossref] [PubMed]
- Hashemi H, Mehravaran S, Rezvan F. Changes in corneal thickness, curvature, and anterior chamber depth during the menstrual cycle. Can J Ophthalmol 2010;45:67-70. [Crossref] [PubMed]
- Bourges JL, Alfonsi N, Laliberté JF, et al. Average 3-dimensional models for the comparison of Orbscan II and Pentacam pachymetry maps in normal corneas. Ophthalmology 2009;116:2064-71. [Crossref] [PubMed]
- Cui J, Zhang X, Hu Q, et al. Evaluation of Corneal Thickness and Volume Parameters of Subclinical Keratoconus Using a Pentacam Scheimflug System. Curr Eye Res 2016;41:923-6. [Crossref] [PubMed]
- OʼDonnell C. Hartwig A, Radhakrishnan H. Comparison of central corneal thickness and anterior chamber depth measured using LenStar LS900, Pentacam, and Visante AS-OCT. Cornea 2012;31:983-8. [Crossref] [PubMed]
- Zhao PS, Wong TY, Wong WL, et al. Comparison of central corneal thickness measurements by visante anterior segment optical coherence tomography with ultrasound pachymetry. Am J Ophthalmol 2007;143:1047-9. [Crossref] [PubMed]
- Dada T, Sihota R, Gadia R, et al. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg 2007;33:837-40. [Crossref] [PubMed]
- Xu BY, Mai DD, Penteado RC, et al. Reproducibility and Agreement of Anterior Segment Parameter Measurements Obtained Using the CASIA2 and Spectralis OCT2 Optical Coherence Tomography Devices. J Glaucoma 2017;26:974-9. [Crossref] [PubMed]
- Chansangpetch S, Nguyen A, Mora M, et al. Agreement of Anterior Segment Parameters Obtained From Swept-Source Fourier-Domain and Time-Domain Anterior Segment Optical Coherence Tomography. Invest Ophthalmol Vis Sci 2018;59:1554-61. [Crossref] [PubMed]
- Kimura S, Morizane Y, Shiode Y, et al. Assessment of tilt and decentration of crystalline lens and intraocular lens relative to the corneal topographic axis using anterior segment optical coherence tomography. PLoS One 2017;12:e0184066. [Crossref] [PubMed]
- Satou T, Kato S, Igarashi A, et al. Prediction of pupil size under binocular open-view settings using the new CASIA2 device. Int Ophthalmol 2019;39:791-6. [Crossref] [PubMed]
- Igarashi A, Shimizu K, Kato S, et al. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg 2019;45:1099-104. [Crossref] [PubMed]
- Fukuda S, Ueno Y, Fujita A, et al. Comparison of anterior segment and lens biometric measurements in patients with cataract. Graefes Arch Clin Exp Ophthalmol 2020;258:137-46. [Crossref] [PubMed]
- Schröder S, Langenbucher A, Schrecker J. Comparison of corneal elevation and pachymetry measurements made by two state of the art corneal tomographers with different measurement principles. PLoS One 2019;14:e0223770. [Crossref] [PubMed]
- Krysik K, Dobrowolski D, Stanienda-Sokol K, et al. Scheimpflug Camera and Swept-Source Optical Coherence Tomography in Pachymetry Evaluation of Diabetic Patients. J Ophthalmol 2019;2019:4532657.
- Xu BY, Penteado RC, Weinreb RN. Diurnal Variation of Optical Coherence Tomography Measurements of Static and Dynamic Anterior Segment Parameters. J Glaucoma 2018;27:16-21. [Crossref] [PubMed]
- Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J Cataract Refract Surg 2006;32:456-9. [Crossref] [PubMed]
- Miranda MA, Radhakrishnan H, O'Donnell C. Repeatability of corneal thickness measured using an Oculus Pentacam. Optom Vis Sci 2009;86:266-72. [Crossref] [PubMed]
- Shoji T, Kato N, Ishikawa S, et al. In vivo crystalline lens measurements with novel swept-source optical coherent tomography: an investigation on variability of measurement. BMJ Open Ophthalmol 2017;1:e000058. [Crossref] [PubMed]
- Hoerauf H, Gordes RS, Scholz C, et al. First experimental and clinical results with transscleral optical coherence tomography. Ophthalmic Surg Lasers 2000;31:218-22. [PubMed]
- Radhakrishnan S, Rollins AM, Roth JE, et al. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 2001;119:1179-85. [Crossref] [PubMed]


