Sun’s total arch replacement and stent elephant trunk with modified branch-first technique for patients with Stanford type A aortic dissection
Introduction
Stanford type A aortic dissection (STAAD), a critical cardiovascular disease, can lead to catastrophic consequences for patients (1,2). For patients with STAAD, surgical procedure is the first-choice treatment (3,4). Despite the continuous advances that have been made in the medical technology for the treatment of STAAD patients, such as hypothermic circulatory arrest, retrograde cerebral perfusion or selective antegrade cerebral perfusion, and the frozen elephant trunk (FET) technique, the surgical procedure still carries a high mortality rate (3.09% to 30%) (4-6). In our hospital, patients with STAAD have been treated by SUN’s procedure (total arch replacement using a tetra-furcate graft and stented elephant trunk implantation) since 2003 (5,7). However, the occurrence of postoperative malperfusion of some critical organs, including the brain, has stayed high (5,7). Consequently, to avoid postoperative neurological malperfusion, a larger proportion of aortic surgeons are attempting new modified procedures.
In this study, we introduce a new modified Sun’s procedure that differs from the classic procedure and the earlier branch-first technique. Furthermore, we examine whether the branch-first Sun’s procedure can more effectively prevent postoperative malperfusion in patients with STAAD than the classic procedure.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3791).
Methods
Study design
This retrospective cohort study analyzed the data of all patients with STAAD who underwent classic Sun’s procedure surgery at Beijing Anzhen Hospital between July, 2017 and November, 2018. The data collected included preoperative variables [age, sex, body mass index (BMI), aortic dissection stage (acute aortic dissection was defined as an occurrence within 14 days), left ventricular ejection fraction (LVEF), aortic insufficiency, mitral insufficiency, tricuspid insufficiency, ascending aortic diameter, aortic sinus diameter, pericardial effusion, alanine transaminase (ALT), aspartate aminotransferase (AST), creatinine (CREA), Troponin I (TnI), left ventricular end diastolic diameter and left ventricular end systolic diameter], intraoperative variables [branch first, cardiopulmonary bypass (CPB) duration, clamp time, hypothermic arrest time, and nasopharyngeal temperature], and postoperative outcome variables (intensive care unit ICU stay, mechanical ventilation time, postoperative dialysis, transfusion of red blood cell, transfusion of plasma, transfusion of platelet, in-hospital death, awake time, and neurological complications.
Neurological complications were evaluated using the definition of distinct neurologic end points (8). Temporary neurological dysfunction was considered when postoperative delirium, agitation, confusion, obtundation, or a transient focal neurologic deficit diagnosed by computed tomography or magnetic resonance imaging were present. Permanent neurologic dysfunction was defined as the occurrence of new neurologic dysfunction following surgical intervention focal injury (stroke), global dysfunction (coma), or evidence of new focal or multiple brain lesions that persisted before discharge from the hospital. The use of these data was approved by the ethics committee of Beijing Anzhen Hospital (Institutional Review Board File 2014019) for clinical research purposes and adhered to the principles outlined in the Declaration of Helsinki, and patients were recruited consecutively if they agreed to provide informed consent.
Patient selection
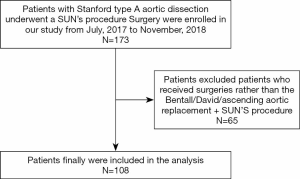
All patients (n=173) with STAAD who underwent classic Sun’s procedure surgery at Beijing Anzhen Hospital between July, 2017 and November, 2018 were included in this retrospective study. Patients with STAAD that involved only the ascending aorta and those with hemodynamic instability that required rapid CPB establishment were excluded from the analysis. After the exclusion of 65 patients who received the Bentall/David/ascending aortic replacement + Sun’s procedure (total arch replacement using a tetra-furcate graft and stented elephant trunk implantation) (5) combined with other cardiac surgeries (e.g., mitral surgery and coronary artery bypass), 108 consecutive patients were included in the final analysis (Figure 1).
Surgical procedure
The surgical procedures involved the concomitant root procedure and total arch replacement, including the branch-first Sun’s procedure (the branch-first group, n=24) and the classic Sun’s procedure (the classic procedure group, n=84).
The branch-first Sun’s procedure requires a Y-shaped graft, which is made while the patient is on the surgical table. The graft comprises a main trunk and a side arm made of two straight Dacron grafts (InterGard, France, or Vascutek, Scotland). The main trunk’s size is based on the width of the innominate artery (usually 12 mm or 14 mm in diameter), and the size of the side arm is determined by the width of the left subclavian artery (LSCA; usually 8 or 10 mm in diameter). The two sections are connected at a 45-degree angle (Figure 2A,B).
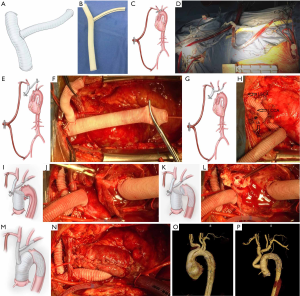
Near infrared reflectance spectroscopy (NIRS) is used on each side of the frontal lobes. The right axillary artery and right common femoral artery are obtained and prepared for cannulation. Median sternotomy is performed to mobilize three arch vessels, and the patient is heparinized (3 mg/kg). Both the right axillary and right femoral arteries are cannulated with a 22 or 24 F cannula depending on vessel diameter. The two cannulas are connected with two heads of a bifurcate arterial line to establish a temporal femoral-axillary bypass (Figure 2C,D). A flowmeter is then attached to the arterial line connecting the axillary cannula.
Without CPB and with a beating heart, the innominate artery (IA) is clamped and transected 1 cm distal to its origin. At this point, a blood supply from the right femoral artery to the IA begins. Continuous suture with 4-0 polypropylene is used to close the stump of the IA. The distal end of the IA is anastomosed with the distal end of the main trunk using 5-0 or 4-0 polypropylene (Figure 2E,F). Then, the left common carotid artery (LCCA) is transected, and the proximal end is closed. A circular fenestration the same size as the LCCA is made in the side arm opposite the LCCA. The distal end of the LCCA is anastomosed to the side arm in an end-to-side fashion. Following the anastomosis, the clamp is moved to the distal aspect of the side arm to establish supply from the femoral-axillary bypass to both the IA and LCCA. Finally, the LSCA is transected. The stump is closed, the side arm is trimmed to a proper length, and the distal end of the LSCA is connected to the side arm in an end-to-end fashion (Figure 2G,H). Up to that point, the three vessels are supplied by the femoral-axillary bypass. During innominate artery reconstruction, the blood flow from the femoral artery to the IA was approximately 600 mL/min, which, after the LCCA was attached to the side arm, raised to 800 mL/min, eventually reaching 1,000 mL/min after all three arch branches had been reconstructed. Flow to the LCCA and LSCA accounted for almost half of the total flow. Meanwhile, there were no significant changes in cerebral oxygen saturation in bilateral cerebral hemispheres according to NIRS monitoring.
Next, the right atrium is cannulated with a two-stage cannula, and the CPB commences. The aortic root or ascending aorta is replaced with a straight graft. The proximal anastomosis is completed first (Figure 2I,J).
When the patient’s temperature drops to 28–30 °C, flow to the femoral artery is stopped. Flow to the right axillary artery is maintained at approximately 7–10 mL/kg/min, keeping the left radial artery at a pressure of 40–60 mmHg. The arch is opened, and an open stent graft (Cronus; MicroPort Medical Co, Ltd, Shanghai, China) is inserted into the arch and descending aorta (Figure 2K,L). Distal aortic anastomosis is performed. Systemic perfusion is restored after the completion of distal anastomosis. The main trunk of the Y-shaped graft is trimmed to a proper length. Its proximal end is beveled and anastomosed to the ascending graft in an end-to-side fashion (Figure 2M,N).
The classic Sun’s procedure has been previously described in detail (5,9,10). The procedure is mostly characterized by the use of right axillary artery cannulation for CPB and selective antegrade cerebral perfusion, as well as the deployment of an FET (Cronus; MicroPort Medical Co., Ltd., Shanghai, China) into the descending aorta, followed by total arch replacement with a four-branched vascular graft. However, the three arch vessels are reconstructed during the CPB, which requires a lower nasopharyngeal temperature for cerebral protection.
The preoperative and postoperative computed tomography angiography (CTA) illustrations are shown in Figure 2O and P.
Statistical analysis
The data were expressed as frequencies and percentages, means ± SDs, or medians and interquartile ranges. T-tests were used to assess whether the continuous variables followed a normal distribution. When variables were not distributed normally, the Wilcoxon rank sum test was applied. Chi-square test or Fisher’s test was applied to compare categorical variates. Univariate regression analysis was used to examine the intraoperative data and postoperative outcomes (CPB duration, clamp time, hypothermic circulatory arrest time, in-hospital death, neurological complications, awake time, postoperative dialysis, ICU stay, and mechanical ventilation time). Based on the recommendations of the STROBE statement, both non-adjusted and adjusted (for age, gender, BMI, acute dissection, LVEF, aortic insufficiency, ascending aortic diameter, pericardial effusion, CREA, and TNI) regression models were employed to evaluate the effect of using the branch-first technique on postoperative outcomes. We selected these confounders on the basis of their associations with the outcomes of interest or a change in effect estimate of more than 10%. All analyses were completed using R statistical software packages (http://www.R-project.org, The R Foundation).
Results
Baseline characteristics of participants
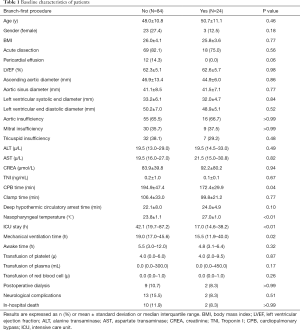
The baseline characteristics of the 108 consecutive patients included in this study are listed in Table 1. The entire cohort had a median age of 48.5±10.8 years and 26 (24.1%) of the patients were female. All patients with preoperative brain malperfusion were considered as contraindication, and the patients with preoperative coronary malperfusion were excluded as they had received concomitant coronary artery bypass; therefore, the mean Troponin I (TnI) was almost normal. The surgical procedures included: the concomitant root procedure (including 34 patients with the Bentall procedure + ascending aortic replacement, and 2 patients with the David procedure + ascending aortic replacement); and single ascending aortic replacement (72 patients). All patients received total arch replacement including 24 patients (22.2%) who received the branch-first Sun’s procedure and 84 patients (77.8%) who received the classic Sun’s procedure. There were 17.9% (15/84) and 25.0% (6/24) cases of acute aortic dissection in the classic and branch-first groups, respectively, but there was no significant difference (P=0.559). Patients in the branch-first group had a significantly shorter CPB duration, ICU stay, and mechanical ventilation time than patients in the classic group. The branch-first group’s nasopharyngeal temperature during hypothermic arrest was significantly higher than that of the classic group.
Full table
Univariate analysis of intraoperative data and postoperative outcomes
Univariate analysis showed that the branch-first method and nasopharyngeal temperature were correlated with better postoperative outcome. The branch-first method may associated with reduced occurrence of neurological complications (OR =0.497; 95% CI: 0.104 to 2.371), postoperative dialysis (OR =0.758; 95% CI: 0.152 to 3.768), and in-hospital deaths (OR =0.637; 95% CI: 0.137 to 3.302), and shortened ICU stay (OR =−23.739; 95% CI: −53.850 to 6.372) and awake time (OR =−1.498, 95% CI: −12.981 to 9.986) (Table 2).

Full table
Multivariate analysis: the effect of the branch-first method on postoperative outcomes
The results of multivariate regression analyses are shown in Tables 3-5. Three models were examined: a simple model (with no adjustments), adjusted model I (adjusted for age, sex, and BMI), and adjusted model II (adjusted for age, sex, BMI, acute dissection, LVEF, aortic insufficiency, ascending aortic diameter, pericardial effusion, CREA, and TNI).

Full table

Full table

Full table
The univariate logistic regression analysis revealed that the branch-first method was associated with better postoperative outcome. After adjustment for confounding variables, the branch-first method was found to be related to a reduction in neurological complications (OR =0.43; 95% CI: 0.08 to 2.25) (Table 3), postoperative dialysis (OR =0.83; 95% CI: 0.13 to 5.35) (Table 4), and in-hospital deaths (OR =0.83; 95% CI: 0.15 to 4.72) (Table 5).
Discussion
With an extremely high in-hospital mortality, the medical management of patients with STAAD needs to be urgently addressed (11-13). Current evidence suggests that aortic surgeons should attempt to operate on all patients with STAAD, which can reduce 1-month mortality by almost 70% (3,4). However, given the high in-hospital surgical mortality rate of patients with STAAD, the surgical techniques need drastic improvement (2).
Since 2003, the team at our hospital has applied Sun’s procedure with all patients with STAAD. Over the same period, the rate of postoperative neurological complications and in-hospital deaths had been a significant reduction (5,7,14). Nevertheless, there is still room for improvement. The procedure’s long operation time and the inevitable hypothermic circulatory arrest resulting from too many anastomoses during CPB means that the risk of postoperative neurological complications and in-hospital death is always present. However, compared with the classic method, the branch-first technique holds some unique advantages: Firstly, under the protection of femoral-axillary bypass, the brachiocephalic arteries reconstruction could be carried out off-pump rather than CPB, which could significantly reduce the CPB and clamp time. Secondly, when brachiocephalic arteries reconstruction was completed, bilateral cerebral perfusion was ensured during subsequent procedure, while increased core temperature during operation may also alleviate adverse effects of deep hypothermic circulatory arrest. Finally, since the brachiocephalic arteries reconstruction was performed first, it could provide more space for arch operation and probably reduce the time pressure of surgeons, which could potentially diminish the risk of anastomotic bleeding.
The earliest prototype of the branch-first technique was reported by Dr. Strauch and Dr. Spielvogel (15,16). They performed bilateral cerebral perfusion in arch surgery instead of unilateral cerebral perfusion, which was more physiological and served as a forerunner of the branch-first technique. This technique requires a lower core temperature of close to 15 °C to protect the brain during the reconstruction of the brachiocephalic vessels. However, this state of deep hypothermia significantly prolongs the process of cooling and rewarming, leading to organ dysfunction and coagulation disorders (17). In 2005, another branch-first technique was adopted by George Matalanis (18,19). This procedure differs to Spielvogel and Strauch’s technique, mainly because it involves establishing a bypass using femoral inflow and moderate hypothermia (28 °C), as well as serial disconnection and reconstruction of each arch branch using a trifurcation arch graft with a perfusion side arm port (20). LeMaire also reported using a similar method to replace the aortic arch (21). This procedure avoids systemic circulatory arrest and deep hypothermia entirely, and the brain is in a state of global perfusion for almost all of the CPB duration. However, they did not establish femoral artery-axillary artery bypass and carried out the reconstruction of the brachiocephalic blood vessels during CPB. Thus, compared with traditional methods, CPB duration or clamping time was not significantly shortened. Compared to their techniques, our procedure possesses some unique features. First, our Y-shaped graft is simple and saves space. Second, by using a temporary femoral-axillary bypass, the three arch branches are reconstructed with a beating heart and without CPB.
As the branch-first technique could provide bilateral cerebral perfusion during hypothermic arrest, theoretically, there should be a clear reduction in neurological complications and awake time in patients with branch-first surgeries. In this study, the results of univariate analysis showed that the branch-first method was associated with better postoperative outcome, including reduced neurological complications and awake time. Three models were established in the multivariate regression analysis of the relationship between the branch-first method and neurological complications, and each model showed a negative relationship. We evaluated the neurological complications using distinct neurologic end points (8). In the entire cohort, 12 patients died in-hospital (2 in the branch-first group and 10 in the classic group), in whom the neurological status and awake time could not be evaluated. As a result, there were some deviations in the final results. After the adjustments to model II, the branch-first method was associated with fewer neurological complications (OR =0.43; 95% CI: 0.08 to 2.25), with a remarkable 57% reduction in the occurrence of neurological complications.
During Sun’s surgery, the spinal cord is one of the areas that needs the most care and protection, and hypothermia plays an important role in increasing its tolerance to ischemia. Throughout the entire process of modified Sun’s procedure with branch-first technique, both bilateral cerebral and subclavian artery perfusion can be provided. All of these aspects effectively ensure blood supply to the spinal cord. As with the current cases and follow-up results, no paraplegia occurred in the branch-first group. In fact, the bilateral cerebral perfusion provided by the branch-first technique allows the surgeon to increase the nasopharyngeal temperature during hypothermic arrest time to the point of normal CPB. However, the distal vessels are always involved in aortic dissection, which can result in malperfusion of several critical organs, especially the spinal cord. A temperature above 28 °C during stent implantation has been reported as a risk factor for spinal cord ischemia (22,23). Consequently, our team set the nasopharyngeal temperature of patients who received the branch-first technique at 28 °C during hypothermic arrest time. In this study, the mean nasopharyngeal temperature for the branch-first group was 27.0±1.0 °C, which was significantly higher than that in the classic group (23.8±1.1 °C, P<0.001). The results of the univariate analysis revealed nasopharyngeal temperature to also be negatively correlated with ICU stay, awake time, in-hospital death, postoperative neurological symptoms, and the occurrence of postoperative dialysis. With each 1 °C increase in nasopharyngeal temperature, ICU stay and awake time were reduced by 11.3 and 2.1 hours respectively. Moreover, the occurrence of postoperative dialysis, postoperative neurological symptoms, and in-hospital death were decreased by 40%, 26%, and 33% respectively.
Some concern may surround the unknown factors in the application of this technique. At present, the flow distribution of the upper and lower body perfusion might be adjusted by the vascular resistance of the human body during the CPB with the use of a single roller pump. The suitability of this adjustment still needs to be confirmed by further research.
Limitations
There were several limitations in this study. First, a retrospective cohort study cannot provide the same validity of evidence as a prospective design. As such, it is difficult to distinguish cause and effect. Moreover, as the study population contained only 108 patients with STAAD who underwent special surgeries at Beijing Anzhen Hospital over the course of 18 months, all STAAD patients could not be included, which might lead to limited statistical power to disclose risk factors. Additionally, the absolute number of 15 events may not be enough to allow adequate multivariable competing analyses. Therefore, a study involving a larger population will be organized in the future.
Conclusions
In conclusion, the branch-first Sun’s procedure has many advantages over the classic technique, including shortened CPB time, reduced occurrence of neurological complications better bilateral cerebral perfusion, and higher nasopharyngeal temperature during hypothermic arrest time. All benefits of the branch-first technique contribute to a quicker post-surgery recovery time for patients.
Acknowledgments
We thank Dr. Zi-Ning Wu for creating the illustrations. We would also like the thank all of the participating physicians who contributed patients to the project.
Funding: This study was supported by the National Key R&D Program of China (No. 2017YFC1308000).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3791
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3791
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3791). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The use of these data was approved by the ethics committee of Beijing Anzhen Hospital (Institutional Review Board File 2014019) for clinical research purposes and adhered to the principles outlined in the Declaration of Helsinki (as revised in 2013), and patients were recruited consecutively if they agreed to provide informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Goebel N, Nagib R, Salehi-Gilani S, et al. One-stage hybrid aortic repair using the frozen elephant trunk in acute DeBakey type I aortic dissection. J Thorac Dis 2018;10:4195-203. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. J Am Coll Cardiol 2010;55:e27-9. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total Arch Replacement Combined With Stented Elephant Trunk Implantation: A New “Standard” Therapy for Type A Dissection Involving Repair of the Aortic Arch?. Circulation 2011;123:971-8. [Crossref] [PubMed]
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, Lack of Evidence, Controversy, and Debate in the Provision and Performance of the Surgery of Acute Type A Aortic Dissection. J Am Coll Cardiol 2011;58:2455-74. [Crossref] [PubMed]
- Ma WG, Zhang W, Wang LF, et al. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg 2016;151:1581-92. [Crossref] [PubMed]
- Ergin MA, Galla JD, Lansman SL, et al. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994;107:788-97; discussion 797-9. [Crossref] [PubMed]
- Ma WG, Zheng J, Dong SB, et al. Sun's procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg 2013;2:621-8. [PubMed]
- Liu ZG, Sun LZ, Chang Q, et al. Should the "elephant trunk" be skeletonized? Total arch replacement combined with stented elephant trunk implantation for Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2006;131:107-13. [Crossref] [PubMed]
- Scholl FG, Coady MA, Davies R, et al. Interval or permanent nonoperative management of acute type A aortic dissection. Arch Surg 1999;134:402-5; discussion 405-6. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Goldstein LJ, et al. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17:615-35. [Crossref] [PubMed]
- Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation 1995;92:II113-21. [Crossref] [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun's procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Strauch JT, Spielvogel D, Lauten A, et al. Technical advances in total aortic arch replacement. Ann Thorac Surg 2004;77:581-89; discussion 589-90. [Crossref] [PubMed]
- Spielvogel D, Strauch JT, Minanov OP, et al. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg 2002;74:S1810-14; discussion S1825-32.
- Khaladj N, Peterss S, Pichlmaier M, et al. The impact of deep and moderate body temperatures on end-organ function during hypothermic circulatory arrest. Eur J Cardiothorac Surg 2011;40:1492-9. [PubMed]
- Matalanis G, Shi WY. An Australian experience with aortic arch replacement: a novel approach without circulatory arrest or deep hypothermia. Heart Lung Circ 2011;20:163-9. [Crossref] [PubMed]
- Matalanis G, Koirala RS, Shi WY, et al. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg 2011;142:809-15. [Crossref] [PubMed]
- Matalanis G, Galvin SD. "Branch-first" continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection. Ann Cardiothorac Surg 2013;2:194-201. [PubMed]
- LeMaire SA, Weldon SA, Coselli JS. Total aortic arch replacement: current approach using the trifurcated graft technique. Ann Cardiothorac Surg 2013;2:347-52. [PubMed]
- Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg 2013;44:1076-82; discussion 1083. [Crossref] [PubMed]
- Etz CD, Luehr M, Kari FA, et al. Selective cerebral perfusion at 28 degrees C--is the spinal cord safe? Eur J Cardiothorac Surg 2009;36:946-55. [Crossref] [PubMed]