
Beta carotene protects H9c2 cardiomyocytes from advanced glycation end product-induced endoplasmic reticulum stress, apoptosis, and autophagy via the PI3K/Akt/mTOR signaling pathway
Introduction
Diabetic cardiomyopathy (DCM) is a cardiac dysfunction commonly underpinned by the formation of protein glycation and advanced glycation end products (AGEs) (1). About 12% of patients with diabetes encounter DCM, which can result in overt heart failure and, consequently, death (2). AGEs are created through an irreversible reaction, named the Maillard reaction, which involves on-enzymatic saccharification (3). Following continuous exposure of proteins and lipids to sugars or short-chain aldehydes with amino groups and/or high oxidative stress levels, glycation and oxidization occur, forming AGEs (4). An increasing amount of research has suggested that the pathogenesis of DCM involves AGEs interacting with the receptor for advanced glycation end products (RAGE) (5). In diabetic patients, AGEs have been shown to induce endothelial dysfunction, myocardial cell apoptosis, and oxidative stress, as well as to upregulate inflammation and to promote extracellular matrix accumulation (6-8).
In patients with diabetes mellitus, nutrient excess induces endoplasmic reticulum (ER) stress in nonvascular tissues (9). When an elevated level of misfolded proteins is present in the ER, the unfolded protein response (UPR) signaling network is triggered, setting off a series of transcriptional and translational events to restore ER homeostasis (10). Emerging evidence has indicated that ER stress can be targeted by AGEs intracellular signaling, ER stress response by AGEs, or AGEs precursors in the pathogenesis of metabolic diseases (11).
Autophagy, which is a degradation process, can transfer intracellular components to lysosomes by autophagosomes (12). Under the condition of excess nutrition, autophagy can mobilize energy to maintain cell survival in periods of nutrient deficiency (13); however, too much autophagy can injure cells (14). Recently, AGEs have been shown to induce cardiomyocyte autophagy by inhibiting the phosphatidyl inositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway via RAGE (15).
AGEs can increase the rate of cardiomyocyte apoptosis and reactive oxygen species (ROS) production (16,17). ROS are essential in AGE-induced human periodontal ligament cells apoptosis and autophagy (18). In rat cardiomyocytes, AGEs can trigger autophagy via the PI3K/AKT/mTOR and ERK signalling pathways and apoptosis via the PI3K/AKT/mTOR and p38/MAPK signalling pathways (19). However, in particular circumstances, autophagy or autophagy-related proteins may eventually cause cells apoptosis or death. Generally, by inhibiting apoptosis, autophagy, and ER stress, cardiomyocyte injuries caused by AGEs may effectively be relieved.
β-carotene (BC), a vitamin A precursor, can be found in a larger number of fruits and vegetables (20). It is widely used as an antioxidant and natural colorant in foods (21,22). Higher BC biochemical status is associated with lower overall, cardiovascular disease, heart disease, stroke, cancer, and other causes of mortality (23). Long-term, low-dose BC treatment could be effective in the treatment of type-2-diabetes and related cardiovascular diseases (24). BC display an antitumoral activity against breast cancer and have the potential to offer a natural strategy for breast cancer chemoprevention and reduce the risk of breast cancer recurrence (25). BC regulates epigenetic modifications for its anti-cancer effects in colon cancer stem cells (26).
However, the benefits of BC in relation to AGE-induced cardiomyocyte injury have not been reported. Therefore, this study aimed to explore the effect of BC on ER stress, ROS production, apoptosis, and autophagy AGE-induced, and explored the potential mechanism.
Methods
Cell culture
H9c2 cells were purchased from American Type Culture Collection (ATCC, Maryland, USA). H9c2 cells were grown in an incubator in Dulbecco’s modified Eagle’s medium (DMEM) complemented with10% fetal bovine serum (FBS) at 37 °C in 5% CO2. Cell were divided four groups: the control group, BC group, AGE-induced group, BC+ AGE-induced group. BC group: H9c2 cells were treated with BC (40 µM) for 24 h. AGE-induced group: H9c2 cells were stimulated with AGEs (200 µg/mL) for 24 h. BC+ AGE-induced group: H9c2 cells were stimulated with AGEs (200 µg/mL) for 24 h, then were treated with BC (40 µM) for 24 h.
Cell viability assay
H9c2 cell viability was detected by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were incubated with MTT solution at 37 °C for 4 h following treatment. Subsequently, the medium was removed, and dimethyl sulfoxide (DMSO) was used to dissolve the formazan crystals. A spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) was used to detect the absorbance at 570 nm.
Measurement of lactate dehydrogenase (LDH) and cardiac troponin-1 (cTn-I) leakage
LDH activity and cTn-I content in the cultured supernatant were measured with diagnostic kits (Roche Diagnostics, Shanghai, China), in accordance with the manufacturer’s protocol.
Flow cytometry
H9c2 cells were treated with BC and/or AGEs for 24 h. They were washed with ice-cold phosphate-buffered saline (PBS) before resuspension in Annexin V binding buffer. Then, incubation with fluorescein isothiocyanate (FITC)-conjugated Annexin V antibody (Cell Signaling Technology, Danvers, USA) and propidium iodide took place for 15 min at room temperature. Data analysis was performed using FlowJo (Tree Star, Ashland, OR, USA).
Western blot
Cells were lysed for 30 min on ice in Radioimmunoprecipitation assay (RIPA) lysis buffer (Solarbio, Beijing, China) containing 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and a protease inhibitor (Roche). BCA kit (Beyotime Institute of Biotechnology, Shanghai, China) was employed to determine protein concentration. The total protein sample was loaded into 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer before being transferred to polyvinylidene difluoride (PVDF) membranes, which were sealed with 5% skim milk at 37 °C for 120 min. The membranes were then incubated with the following primary antibodies: Bcl-2, Bcl-2-associated X protein (Bax), Cleaved caspase-3, ATF4, GRP78, CHOP, Beclin4, p62, LC3I, LC3II, p-P13K, p-AKT, p-mTOR and β-actin (all purchased from Abcam, Cambridge, MA, USA) at 4 °C overnight. After that, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The signals were visualized with the enhanced Horseradish Peroxidase (Pierce, Rockford, IL, USA). An automatic digital gel image analysis system Bio-Rad CFX-96 (Bio-Rad, CA, USA) was used to determine and analyze the density of the bands.
Measurement of intracellular oxidants and antioxidant enzyme activities
The ROS production was measured with dichloro-dihydro-fluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology, China), in accordance with the instructions of the manufacturer. Flow cytometry was performed to measure fluorescent density.
According to the manufacturer’s instructions, the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and Glutathione peroxidase (GSH-Px) were measured with commercially available MDA kits, SOD assay kit and GSH-Px kit (Jiancheng Bioengineering Institute, Nanjing, China), respectively.
Immunofluorescence staining
H9c2 cells were cultured on glass coverslips in a 12-well chamber and treated with BC and/or AGEs. Subsequently, H9c2 cells were incubated overnight with anti-microtubule-associated protein 1 light chain 3 (LC3) antibody, and the corresponding anti-rabbit IgG secondary antibodies was incubated at 37 °C for 1 h. An Olympus DX51 fluorescence microscope (Olympus, Tokyo, Japan) was used to make observations. Data analysis was performed inimage6.0 (Media Cybernetics, USA).
Statistical analyses
Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). The results were expressed as mean ± standard deviation (SD). One-way ANOVA was performed to analyze statistical significance. A P value of <0.05 was considered statistically significant.
Results
BC protects against AGE-induced cell death
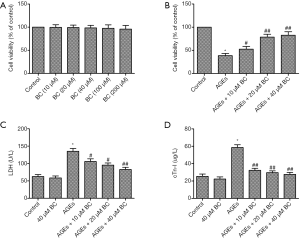
To gain insight into the cytotoxicity of BC on cardiomyocytes, an MTT assay was performed to measure the viability of H9c2 cells exposed to BC at different doses. As shown in Figure 1A, BC up to 200 µM had no effect on cell death. In addition, the viability of AGE-induced H9c2 cells was decreased, whereas a significant, dose-dependent increase was observed in the viability of BC-treated cells (Figure 1B). LDH and cTn-I, the release of which indicates cellular injury, were markedly upregulated by AGEs in comparison with the control group. However, pretreatment with BC dose-dependently decreased AGE-induced increase of LDH and cTn-I (Figure 1C,D). These results indicate that BC pretreatment inhibited AGE-induced cell death. Therefore, a dose of 40 µM BC was used for in the following experiments.
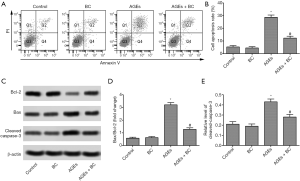
BC attenuates AGE-induced cell apoptosis
H9c2 cell apoptosis was upregulated in the AGEs group, while H9c2 cells apoptosis was downregulated in the AGEs+ BC group compared with the AGEs group (Figure 2A,B). Moreover, BC treatment caused a significant reduction in proapoptotic Bax expression but upregulated anti-apoptotic B-cell lymphoma-2 (Bcl-2) expression, which was accompanied by downregulated caspase3 activity compared with the AGE-induced group (Figure 2C,D,E). These results suggested that BC inhibited early stage apoptosis AGE-induced.
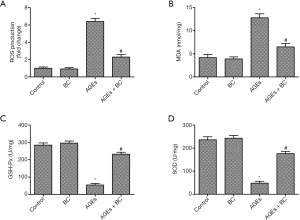
BC suppresses AGE-induced cell oxidative stress
The levels of ROS were enhanced in the AGEs group. However, the results showed that BC significantly inhibited AGE-induced intracellular ROS production (Figure 3A). The oxidative stress biomarker MDA decides the extent of membrane lipid peroxidation, and excessive ROS can be eliminated by free radical scavenging enzymes, such as SOD and GSH-Px. As shown in Figure 3B,C,D, AGE-stimulated cells displayed increased MDA production with decreased levels of GSH-Px and SOD; although, these effects were essentially relieved by BC. Taken together, these results indicate that pretreatment with BC can suppress AGE-induced oxidative stress in H9c2 cells.
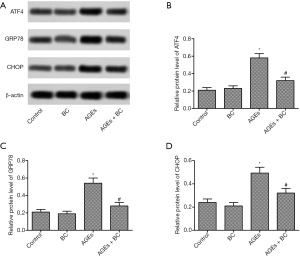
BC alleviates AGE-induced elevation of ER stress
To further explore the role of AGEs in ER stress. ER stress-related proteins were detected by Western blot. AGEs induced a significant increase in the protein expressions levels of activating transcription factor 4 (ATF4), glucose-regulated protein 78 (GRP78), and CCAAT/enhancer-binding protein homologous protein (CHOP) (Figure 4), whereas BC treatment markedly decreased the levels of these proteins. These results indicate that BC could alleviate AGE-induced ER stress in H9c2 cells.
BC inhibits AGE-induced autophagy
Autophagy is also a necessary mechanism involved in different cardiac injuries. To understand whether BC affects AGE-induced myocardial cell autophagy, the autophagy-related proteins were detected by Western blot. There was a marked increase in the protein expression levels of Beclin1, but a decrease inp62, with AGEs treatment. However, BC treatment effectively corrected these abnormalities (Figure 5A,B,C). Moreover, BC treatment significantly decreased AGE-induced elevation of LC3II/LC3I ratio and numberLC3-labeled puncta in each cell (Figure 5D,E,F). These results indicate that BC inhibited AGE-induced autophagy in H9c2 cells.
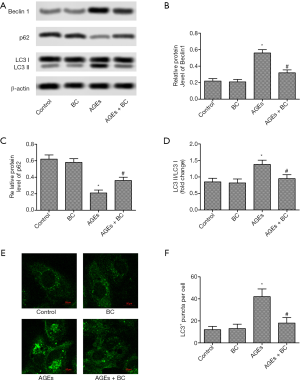
BC activates AGE-induced inhibition of the PI3K/Akt/mTOR signaling pathway
PI3K/Akt/mTOR signaling pathway oversees vital activity in regulating cell apoptosis and autophagy. As shown in Figure 6, treatment with AGEs significantly decreased the phosphorylation of PI3K, Akt, and mTOR. Interestingly, BC treatment notably upregulated the expression of phosphorylated PI3K, Akt, and mTOR. These results indicate that BC can activate AGE-induced inhibition of the PI3K/Akt/mTOR signaling pathway.
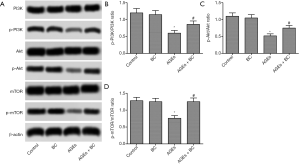
PI3K/Akt/mTOR is essential in BC protection of AGE-induced cardiac injuries
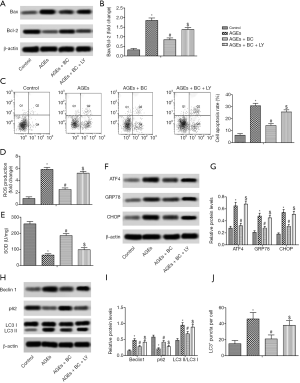
To determine whether BC activation of the PI3K/Akt/mTOR pathway contributes to its cardiac protection, the PI3K signaling inhibitor LY294002 was further studied. The results showed that by inhibiting the PI3K/Akt/mTOR pathway withLY294002 there was a partial reversal in the downregulation of apoptosis, oxidative stress, ER stress, and autophagy induced by BC in H9c2 cells (Figure 7). These findings indicated that BC offers protection against AGE-induced cardiac damageviaPI3K signaling.
Discussion
BC is an active antioxidant and the major dietary source of provitamin A. Previous research has showed that low serum carotenoid levels are indicative of a higher density of lipoproteins or inflammation, each of which are novel risk factors for coronary heart disease (27). Numerous studies have showed that BC has a potential role in the prevention of cardiovascular disease (28). A recent study on human lung cancer A549 cells demonstrated that Vitamin A downregulates the expression of RAGE byactivatingp38 MAPK and NF-κB in an oxidant-dependent manner (29). In this study, we first found that BC could protect AGE-induced cardiac dysfunction.
AGEs are key components in cardiac aging and fibrosis. RAGE and the TGF-β/Smad signaling pathway may feature in the cardiac aging process caused by AGE (30). The accumulation of AGEs is the main mechanism of cardiomyocyte aging and plays a significance role in metabolic diseases (31-33). AGE-RAGE activity is crucial in cardiac dysfunction and the onset of DCM (34). This study found that AGEs could induce cell death in H9c2 cells, as demonstrated by the marked loss of viability, the release of LDH and cTn-I, and the induction of cell apoptosis. However, BC treatment effectively corrected these abnormalities. In this research, these findings exhibited that BC could protect against AGE-induced cardiac cell death.
The enhancement of oxidative stress is central in the pathogenesis of DCM though RAGE activation (35,36). Chrysin, as a PPAR-γ agonist, relieves myocardial injury by inhibiting AGE-RAGE-mediated oxidative stress and inflammation in diabetic rats (37). Mangiferin inhibits AGEs by deactivating NF-κB and had displayed an anti-oxidative effect in streptozotocin and rats with DCM on a high-fat diet-DCM rats (38). By pre-treating K562 cells with BC, the impact of oxidative damage resulting from H2O2 and cell death was altered (39). Oxidative stress was reduced after beta-carotene supplementation in chronic lead poisoning (40). In this research, the function of BC on AGE-induced oxidative stress in H9c2 cells was investigated. This result displayed that there was a significant increase in ROS generation in the AGE-induced H9c2 group. Pre-treatment with BC mitigated the ROS production and the inhibitory functions of AGEs on the synthesis of antioxidases.
Previous studies have indicated that inhibitors of advanced glycation acting as potent ER stress modulators with beneficial effects in restoring ER homeostasis (11). More specifically, an evidence has shown that advanced oxidation protein products trigger podocyte apoptosis through induction of ER stress (41). A similar study on Alzheimer’s disease showed that through ER stress, AGEs counteracted the neuroprotective influence of Sirt1, resulting in the death of neuronal cells (42). Subsequent studies showed that AGEs directly induced ER stress in human aortic endothelial cells and were significantly involved with apoptosis of endothelial cells (43). At the same time, the ER stress pathway through activation of Nox4 by integrins alpha1beta1 plays a key role in 3DG-collagen-induced caspase-3 activation (44). Our results indicated that AGEs can adversely affect ER function, leading to pathogenic ER stress, as demonstrated by the upregulation of ATF4, GRP78, and CHOP. BC treatment also relieved AGE-induced ER stress, which shows that it may offer protection effect.
Autophagy is also a major regulator of homeostasis and function of the heart (45). Autophagy especially contributes to cardiac ischemia, hypertension and diabetes by interacting with ROS generated in the ER and mitochondria (46). Surprisingly, autophagy is inhibited by high glucose levels (47). Furthermore, decreased autophagy is an adaptive response that can limit cardiac dysfunction in type 1 diabetes by upregulating autophagy and the alternative effects of autophagy (48). We observed that autophagy was activated by AGEs in H9c2 cells. BC treatment reversed the induction of autophagy-related proteins, indicating that the inhibition of autophagy also plays a role in the protective effect of BC on AGEs-induced cardiac dysfunction.
The PI3K/Akt/mTOR signaling pathway, a vital intracellular mediator, is essential in regulating survival, apoptosis, and autophagy in cells (49-51). It is reported that the autophagy and apoptosis are activated via the inhibition of the PI3K/Akt/mTOR signaling pathway in prostate cancer stem cells (52). Likewise, ER stress may also be induced by inhibiting the PI3K/Akt/mTOR signaling pathway (53). We observed the marked downregulation of phosphorylated PI3K (p-PI3K), phosphorylated Akt (p-AKT), and phosphorylated mTOR (p-mTOR) following exposure to AGEs, and their partial restoration after BC treatment. Moreover, LY29022-mediated suppression of PI3K/Akt/mTOR signaling in part reversed the effects exhibited by BC on apoptosis, oxidative stress, ER stress, and autophagy. These results indicated that BC protects H9c2 cardiomyocytes from AGE-induced by activating the PI3K/Akt/mTOR pathway.
In summary, BC was observed to protect H9c2 cardiomyocytes from AGE-induced apoptosis, ER stress, and autophagy. Our study provides new insights into the potential application of BC in the treatment of DCM or other cardiac dysfunction and provides a basis for further exploration into the sequence and interaction between the biological processes involved.
Acknowledgments
Funding: This work is supported by Henan Science and Technology Department Project (2011-873).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3768
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3768). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang T, Hu X, Cai Y, et al. Metformin protects against hyperglycemia-induced cardiomyocytes injury by inhibiting the expressions of receptor for advanced glycation end products and high mobility group box 1 protein. Mol Biol Rep 2014;41:1335-40. [Crossref] [PubMed]
- Gazzaruso C, Coppola A, Montalcini T, Falcone C. Anti-diabetic agents and heart health: how to use new diabetes medications in a global strategy for the prevention of cardiovascular complications in type 2 diabetes. Ann Transl Med 2018;6:195. [Crossref] [PubMed]
- Yamagishi S, Takeuchi M, Inagaki Y, et al. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int J Clin Pharmacol Res 2003;23:129-34. [PubMed]
- Deluyker D, Evens L, Bito V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: focus on high molecular weight AGEs. Amino Acids 2017;49:1535-41. [Crossref] [PubMed]
- Yamagishi SI, Nakamura N, Matsui T. Glycation and cardiovascular disease in diabetes: A perspective on the concept of metabolic memory. J Diabetes 2017;9:141-8. [Crossref] [PubMed]
- Bodiga VL, Eda SR, Bodiga S. Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev 2014;19:49-63. [Crossref] [PubMed]
- Yuan Q, Zhou QY, Liu D, et al. Advanced glycation end-products impair Na+/K+-ATPase activity in diabetic cardiomyopathy: role of the adenosine monophosphate-activated protein kinase/sirtuin 1 pathway. Clin Exp Pharmacol Physiol 2014;41:127-33. [Crossref] [PubMed]
- Nozynski J, Zakliczynski M, Konecka-Mrowka D, et al. Advanced glycation end products and lipofuscin deposits share the same location in cardiocytes of the failing heart. Exp Gerontol 2013;48:223-8. [Crossref] [PubMed]
- Bretón-Romero R, Weisbrod RM, Feng B, et al. Liraglutide Treatment Reduces Endothelial Endoplasmic Reticulum Stress and Insulin Resistance in Patients With Diabetes Mellitus. J Am Heart Assoc 2018;7:e009379. [Crossref] [PubMed]
- Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 2015;10:173-94. [Crossref] [PubMed]
- Piperi C, Adamopoulos C, Dalagiorgou G, et al. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab 2012;97:2231-42. [Crossref] [PubMed]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 2010;221:3-12. [Crossref] [PubMed]
- Kim KH, Lee MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol 2014;10:322-37. [Crossref] [PubMed]
- Fulda S. Autophagy and cell death. Autophagy 2012;8:1250. [Crossref] [PubMed]
- Hou X, Hu Z, Xu H, et al. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol 2014;13:78. [Crossref] [PubMed]
- Xie Y, Xiao F, Luo L, et al. Activation of autophagy protects against ROS-mediated mitochondria-dependent apoptosis in L-02 hepatocytes induced by Cr(VI). Cell Physiol Biochem 2014;33:705-16. [Crossref] [PubMed]
- Rojas A, Mercadal E, Figueroa H, et al. Advanced Glycation and ROS: a link between diabetes and heart failure. Curr Vasc Pharmacol 2008;6:44-51. [Crossref] [PubMed]
- Mei YM, Li L, Wang XQ, et al. AGEs induces apoptosis and autophagy via reactive oxygen species in human periodontal ligament cells. J Cell Biochem 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hu P, Zhou H, Lu M, et al. Autophagy Plays a Protective Role in Advanced Glycation End Product-Induced Apoptosis in Cardiomyocytes. Cell Physiol Biochem 2015;37:697-706. [Crossref] [PubMed]
- Berman J, Zorrilla-López U, Farré G, et al. Nutritionally important carotenoids as consumer products. Phytochemistry Reviews 2015;14:727-43. [Crossref]
- Coronel-Aguilera CP, Martín-González MFS. Encapsulation of spray dried β-carotene emulsion by fluidized bed coating technology. LWT - Food Science and Technology 2015;62:187-93. [Crossref]
- de Paz E, Martín Á, Rodríguez-Rojo S, et al. Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocolloids 2012;26:17-27. [Crossref]
- Huang J, Weinstein SJ, Yu K, et al. Serum Beta Carotene and Overall and Cause-Specific Mortality. Circ Res 2018;123:1339-49. [Crossref] [PubMed]
- Csepanyi E, Czompa A, Szabados-Furjesi P, et al. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int J Mol Sci 2018;19:1132. [Crossref] [PubMed]
- Mokbel K, Mokbel K. Chemoprevention of Breast Cancer With Vitamins and Micronutrients: A Concise Review. In Vivo 2019;33:983-97. [Crossref] [PubMed]
- Kim D, Kim Y, Kim Y. Effects of β-carotene on Expression of Selected MicroRNAs, Histone Acetylation, and DNA Methylation in Colon Cancer Stem Cells. J Cancer Prev 2019;24:224-32. [Crossref] [PubMed]
- Kritchevsky SB. beta-Carotene, carotenoids and the prevention of coronary heart disease. J Nutr 1999;129:5-8. [Crossref] [PubMed]
- Gerster H. Potential role of beta-carotene in the prevention of cardiovascular disease. Int J Vitam Nutr Res 1991;61:277. [PubMed]
- de Bittencourt Pasquali MA, Gelain DP, Zeidán-Chuliá F, et al. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell Signal 2013;25:939-54. [Crossref] [PubMed]
- Min F, Wang J, Li S, et al. Advanced glycation end-products accelerate the cardiac aging process through the receptor for advanced glycation end-products/transforming growth factor-β-Smad signaling pathway in cardiac fibroblasts. Geriatr Gerontol Int 2016;16:522-7. [Crossref] [PubMed]
- Simm A. Protein glycation during aging and in cardiovascular disease. J Proteomics 2013;92:248-59. [Crossref] [PubMed]
- Robert L, Labat-Robert J. Role of the Maillard reaction in aging and age-related diseases. Studies at the cellular-molecular level. Clin Chem Lab Med 2014;52:5-10. [Crossref] [PubMed]
- Cárdenas-León M, Díaz-Díaz E, Argüelles-Medina R, et al. Glycation and protein crosslinking in the diabetes and ageing pathogenesis. Rev Invest Clin 2009;61:505-20. [PubMed]
- Ma H, Li SY, Xu P, et al. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) up-regulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med 2009;13:1751-64. [Crossref] [PubMed]
- Lee TW, Kao YH, Lee TI, et al. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol 2014;173:236-41. [Crossref] [PubMed]
- Rajesh M, Bátkai S, Kechrid M, et al. Cannabinoid 1 Receptor Promotes Cardiac Dysfunction, Oxidative Stress, Inflammation, and Fibrosis in Diabetic Cardiomyopathy. Diabetes 2012;61:716-27. [Crossref] [PubMed]
- Rani N, Bharti S, Bhatia J, et al. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact 2016;250:59. [Crossref] [PubMed]
- Hou J, Zheng D, Fung G, et al. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can J Physiol Pharmacol 2016;94:332-40. [Crossref] [PubMed]
- Akçakaya H, Tok S, Dal F, et al. β-carotene treatment alters the cellular death process in oxidative stress-induced K562 cells. Cell Biol Int 2017;41:309-19. [Crossref] [PubMed]
- Kasperczyk S, Dobrakowski M, Kasperczyk J, et al. Beta-carotene reduces oxidative stress, improves glutathione metabolism and modifies antioxidant defense systems in lead-exposed workers. Toxicol Appl Pharmacol 2014;280:36-41. [Crossref] [PubMed]
- Rong G, Tang X, Guo T, et al. Advanced oxidation protein products induce apoptosis in podocytes through induction of endoplasmic reticulum stress. J Physiol Biochem 2015;71:455-70. [Crossref] [PubMed]
- Ko SY, Ko HA, Chu KH, et al. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer's Disease. Plos One 2015;10:e0143345. [Crossref] [PubMed]
- Adamopoulos C, Farmaki E, Spilioti E, et al. Advanced glycation end-products induce endoplasmic reticulum stress in human aortic endothelial cells. Clin Chem Lab Med 2014;52:151-60. [Crossref] [PubMed]
- Loughlin DT, Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One 2010;5:e11093. [Crossref] [PubMed]
- Sciarretta S, Maejima Y, Zablocki D, et al. The Role of Autophagy in the Heart. Annu Rev Physiol 2018;80:1-26. [Crossref] [PubMed]
- Mei Y, Thompson MD, Cohen RA, et al. Autophagy and oxidative stress in cardiovascular diseases. Biochim Biophys Acta 2015;1852:243-51. [Crossref] [PubMed]
- Kobayashi S, Xu X, Chen K, et al. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 2012;8:577-92. [Crossref] [PubMed]
- Xu X, Kobayashi S, Chen K, et al. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 2013;288:18077-92. [Crossref] [PubMed]
- Sanghera KP, Mathalone N, Baigi R, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci 2011;47:145-53. [Crossref] [PubMed]
- Park KR, Nam D, Yun HM, et al. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett 2011;312:178-88. [Crossref] [PubMed]
- Wu YT, Tan HL, Huang Q, et al. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 2009;5:824-34. [Crossref] [PubMed]
- Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett 2014;343:179-89. [Crossref] [PubMed]
- Chen XL, Fu JP, Shi J, et al. CXC195 induces apoptosis and endoplastic reticulum stress in human hepatocellular carcinoma cells by inhibiting the PI3K/Akt/mTOR signaling pathway. Mol Med Rep 2015;12:8229-36. [Crossref] [PubMed]


