
Critical care echocardiography: diagnostic or prognostic?
Echocardiography is a widespread, noninvasive, portable and powerful imaging modality which provides unparalleled anatomical and functional information on both the heart and great vessels. When used in intensive care medicine, echocardiography may be focused rather than comprehensive, is both performed and interpreted around-the-clock by the front-line physician in light of the clinical context and ongoing therapy, and frequently results in a broad and direct therapeutic impact (1). These specificities have led to define critical care echocardiography (CCE) as examinations performed and interpreted by the intensivist to make diagnoses and guide therapeutic management of the critically ill patient with cardiovascular or respiratory compromise (2). Dedicated training programs have been validated over the past decade. Despite the pivotal role gained by CCE in the intensive care unit (ICU) settings, studies assessing its impact on patient-centered outcome are scarce.
In this context, contributions such as that reported by Lan et al. (3) in the journal should be encouraged. Authors have used a large open-access database containing anonymous health-related data of 46,520 critically ill patients admitted to the Beth Israel Deaconess Medical Center in Boston (MA, USA) from 2001 to 2012 (4). Using a propensity score analysis to match patients who were assessed using CCE during the first 24 h of the septic shock onset (n=1,289) and those who were not (n=1,289), authors showed that 28-day mortality was significantly lower in the ultrasound group (33.2% vs. 37.7%: P=0.019). Improved 28-day mortality in patients assessed with CCE (OR: 0.83; 95% CI: 0.73–0.95: P=0.005) was confirmed by sensitivity analyses in the subgroup of patients who underwent a single echocardiography examination (n=2,464; OR: 0.82; 95% CI: 0.72–0.94; P=0.004) and when excluding patients monitored using either a right-heart catheter or transpulmonary thermodilution (n=2,485; OR: 0.87; 95% CI: 0.76–0.99: P=0.034) (3). These results confirm those reported by the previous contribution of Feng et al. (5) based on the same Medical Information Mart for Intensive Care (MIMIC)-III database in 6,361 patients with sepsis. Using sophisticated multivariate statistical analysis, these authors showed a significant beneficial effect of CCE assessment on 28-day mortality when performed less than 24 h before the first ICU admission (51.3%) or during ICU stay. Propensity score-matched mortality rates for CCE and no CCE groups were 25% and 30%, respectively, and adjusted odds ratio was 0.78 (95% CI: 0.68–0.90; P<0.001) (5).
What are the factors driving the performance of CCE and do they influence its association with outcome?
Scientific Societies of Intensive Care Medicine have defined the use of CCE and training content to achieve different levels of competence (2). Noticeably, CCE is suggested as the preferred modality to initially evaluate the type of shock as opposed to more invasive technologies (week recommendation; quality of experience moderate; level of evidence: B) (6). Intuitively, the therapeutic impact of CCE—hence potential benefit on patient course—heavily depends on the contextual indication of the hemodynamic assessment which will determine both its diagnostic yield and potential additional value (Figure 1). Not surprisingly, the presence of congestive heart failure was the most discriminant relative influence factor to predict the likelihood of CCE performance in the propensity score model developed by Feng et al. (5). In this study, CCE was associated with a significantly lower 28-day mortality in septic patients from the MIMIC-III database, irrespective of the five models tested (5). In the present contribution, Lan et al. (3) reported that after matching, septic patients from the same database more frequently received right-heart catheterization but not transpulmonary thermodilution when assessed using CCE. This suggests that those specific patients, who were sicker than their counterparts before matching, required a comprehensive hemodynamic assessment and monitoring according to their clinical presentation.
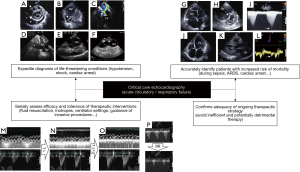
A large-scale study was recently performed on the Nationwide Inpatient Sample (NIS) which represents the largest publicly available database in the United States, with approximately 8 million hospitalizations in a sample of 20% community hospitals (7). The use of echocardiography was associated with a significant lower adjusted odds of hospital mortality in approximately half of all inpatients who were hospitalized for five of the leading six admission diagnoses (7). All of those, namely sepsis, congestive heart failure, acute myocardial infarction, acute cerebrovascular disease and cardiac dysrhythmia, are conditions which are frequently encountered in ICU patients. Overall, the potential impact of echocardiography on patient outcome is presumably influenced by the clinical presentation and suspected underlying condition, especially when severe and involving the cardiovascular system.
Could CCE predict mortality in ICU patients?
In a small sample size study, Heidenreich et al. (8) reported that transesophageal echocardiography (TEE) diagnosis was an independent predictor of mortality in ICU patients who were hemodynamically assessed for hypotension. Authors distinguished three groups based on the mechanism of hypotension identified by TEE: non-ventricular cardiac limitation in cardiac output (e.g., pericardial or valvular disease) (group 1); depressed ventricular systolic function (group 2); vasoplegia (hyperkinetic left ventricle) and/or hypovolemia (group 3). Patients from group 1 had a significantly higher survival rate (81%) than those of group 2 (41%) and group 3 (44%; P=0.03). Not surprisingly, direct therapeutic impact resulting from TEE assessment was more frequent in group 1 than in group 2 and 3 (75% vs. 41% vs. 33%: P=0.03), including surgery (56% vs. 7% vs. 6%: P=0.0001) (8). CCE expedites diagnosis of imminent life-threatening conditions which require immediate intervention, such as tamponade, acute and severe valvular regurgitation, thrombus-in-transit associated with massive pulmonary embolism, or acute aortic disease with extravasation signs (Figure 1). Nevertheless, these findings are occasional and tend to overestimate the overall diagnostic ability and potential prognostic impact of CCE in ICU patients.
Echocardiography is recommended during the cardiopulmonary resuscitation of cardiac arrest to help identifying potentially treatable causes and to assess myocardial contractility (Figure 1). CCE appears to predict mortality during cardiopulmonary resuscitation since the absence of cardiac activity on CCE had a pooled negative likelihood ratio of 0.18 for predicting the return of spontaneous circulation (9). In 793 out-of-hospital cardiac arrests, Gaspari et al. (10) reported that only 0.6% of patients with absence of initial cardiac activity on CCE survived to hospital discharge.
In patients receiving protective mechanical ventilation for the acute respiratory distress syndrome (ARDS), the identification of a severe acute cor pulmonale by TEE, as defined by the conjunction of a marked dilatation of the right ventricle which end-diastolic area exceeds that of the left ventricle in the long axis view of the heart and a paradoxical septal motion in the short-axis view, is independently associated with increased hospital mortality (11). In addition to its valuable ability to semi-quantitatively assess left ventricular filling pressure in patients presenting with acute respiratory failure (i.e., rule in/rule out cardiogenic pulmonary edema), CCE depicts hemodynamic consequences of both associated pulmonary microvascular involvement and positive-pressure ventilation on right ventricular function which appear prognostic when marked (Figure 1).
Although association does not prove causality, taken together these findings suggest that CCE may help the front-line clinician to identify mortality risk and take preemptive action to improve patient outcome in certain clinical settings, other than sepsis. Nevertheless, the generalization of this sound clinical approach may not hold true in all ICU patients (12).
Which CCE-induced changes in therapy could influence mortality?
In the two recent studies reporting an improved 28-day mortality in septic patients from the MIMIC-III data base who were initially assessed using CCE, a significant therapeutic impact presumably related to initial noninvasive hemodynamic assessment has been described (3,5). When compared to patients who were not evaluated by CCE, those patients who underwent an echocardiography assessment during the initial management of sepsis received significantly more fluids throughout the first three days of hospitalization (5), more inotropes (3,5), and shorter duration of vasopressors (5). In contrast, the duration of mechanical ventilation was not statistically different between groups (3,5).
In a recent randomized controlled trial (RCT) performed in pediatric septic shock, the proportion of children with successful shock reversal was significantly higher (89% vs. 67%: P=0.01) and the duration of shock shorter (3.3 vs. 4.5 days: P=0.01) in those who were serially assessed with transthoracic echocardiography (TTE) to determine both the volume status and myocardial function (13). In keeping with these results, lactate normalized faster in the intervention arm. Interestingly, earlier and higher fluid volume resuscitation was administered in children monitored with CCE, but cumulative fluid volume was significantly lower at 24 h when compared to controls (13). Moreover, inotropes were more frequently used in the experimental group (89% vs. 67%: P=0.01) with earlier starting time (12 vs. 24 h: P=0.01) than in the control group. Interestingly, mortality attributed to unresolved shock was significantly lower in children monitored with CCE (38.5% vs. 88.2%: P=0.006) (13). Similar findings were reported by Kanji et al. (14) in adult patients with vasopressor-dependent shock in a before/after study where patients were hemodynamically assessed using focused echocardiography (intervention) in addition to standard of care (control period).
In guiding the therapeutic management of patients with cardiopulmonary compromise, CCE has a significant therapeutic impact which may secondarily improve patient-centered outcome. Specific therapy such as additional fluid loading or inotrope initiation may be delivered earlier and adapted serially when guided by CCE monitoring (Figure 1). This may avoid unnecessary fluids or drugs and guide therapeutic changes to better take into account the complex course of ICU patients, especially when sustaining septic shock.
Why therapeutic impact does not translate into prognostic improvement?
In Cardiology settings, appropriate use criteria have been established to optimize the rational use of TTE and potentially help the decision making of attending physicians. TTE examinations fulfilling these criteria appear to result in a more frequent therapeutic impact than when performed for inappropriate indications (86.7% vs. 14.1%: P<0.0001) (15). Despite mostly appropriate examinations (91.8%), Matulevicius et al. (16) showed that nearly half of TTEs resulted in continuation of ongoing care and less than one-third (31.8%) induced therapeutic changes. When confirming that initiated empiric therapy is adequate in ICU patients, CCE is as informative as when it provides additional information, since it avoids unnecessary and potentially detrimental treatment (e.g., excessive fluid resuscitation in non-responders) (Figure 1). In 137 ventilated patients assessed using TEE for septic shock, hemodynamic assessment confirmed the adequacy of initiated therapy in 27% of the cases (17). According to clinical settings, the respective proportion of therapeutic changes and validation of ongoing management following CCE examination is expected to vary greatly, so as resulting potential impact on outcome.
Early recognition of hemodynamic disorders and guidance to determine appropriate initial therapy aimed at reducing the duration of hypotension and tissue hypoperfusion have been shown to decrease morbidity and mortality in patients sustaining septic shock (18). Accordingly, the timing of hemodynamic assessment, including when using CCE, is crucial to consider. In patients presenting with septic shock, CCE is typically performed within the first hours following ICU admission, after initial fluid resuscitation and stabilized blood pressure under vasopressor support (17), as in the present contribution (3). CCE allows early identification of distinct cardiovascular phenotypes in patients admitted to the ICU for septic shock (19). Whether this strategy will select the subset of patients whose outcome could be improved by a specific treatment (e.g., inotropes) remains to be determined. To address this hypothesis, CCE will be used to select eligible patients with associated septic cardiomyopathy for their potential enrollment in a RCT comparing the effects of dobutamine vs. placebo on sepsis-induced organ dysfunctions (NCT04166331). In contrast, uniformly administered inodilator increases the frequency of adverse effects and fails to improve mortality when compared to placebo (20). Interestingly, early CCE assessment promises to provide valuable information to guide tailored resuscitation as soon as the diagnosis of sepsis is raised in the Emergency Department (21). Whether this precocious information may alter the early Surviving Sepsis Campaign bundle and patient-centered outcome remains to be investigated. As opposed to common practice in Cardiology, CCE is currently more considered and used to monitor both the efficacy and tolerance of therapeutic interventions rather than as a punctual diagnostic imaging modality (Figure 1) (22). Accordingly, future studies should take into account this relevant and rapidly spreading clinical practice (3).
When compared with the sole clinical assessment, hemodynamic monitoring provides relevant additional information with a potential impact on therapeutic decisions. Nevertheless, for this new information to translate into an improvement of outcome, hemodynamic assessment should be accurate, adequately interpreted and associated with a standardized therapeutic algorithm (17). In contrast, if the additional information is interpreted or applied inappropriately, resulting therapeutic interventions may prove ineffective or harmful, and outcome will not be improved or even may be worsened (23). Accordingly, the measurement of routinely used hemodynamic parameters should be reproducible and adequate training of intensivists is key, irrespective of the targeted level of competence in CCE (24). Finally, according to the clinical scenario, the use of TEE rather than TTE may alter resulting therapeutic impact due to its higher diagnostic capacity for the identification of certain conditions (e.g., extrapericardial tamponade after cardiac surgery), hence potential influence of CCE on outcome.
Are RCTs to establish the prognostic role of CCE necessary in ICU patients?
Currently, no definitive RCT aimed at assessing the prognostic impact of CCE in ICU patients has been fully conducted. Reasons are numerous (Table 1). The only published RCT evaluated the influence of point-of-care ultrasonography on mortality of patients presenting to the Emergency Department with undifferentiated (i.e., without a clearly evident cause) non traumatic hypotension or shock (25). This study failed to find any benefit to perform early point-of-care ultrasonography on patient-centered outcome, when compared to standard of care. Interestingly, this RCT was interrupted prematurely at interim analysis because of slow recruitment due to concerns about randomization to the control group from physicians and perceived futility of continuing (25). In addition to its lack of power, this study suffered from substantial limitations: exclusion of clear mechanisms of hypotension or shock (potential bias), absence of specific clinical questions to address (unknown presumptive diagnoses), and lack of associated predefined therapeutic algorithm (random therapeutic impact). Not surprisingly, the volume of fluid resuscitation and proportion of patients receiving inotropes were not statistically significant between groups (25).

Full table
Wrap-up
Since echocardiography is the most ubiquitous, portable, cost-effective and well-tolerated imaging modality with an infinitesimal risk-to-benefit ratio, the high therapeutic impact resulting from its routine use in ICU patients may be interpreted as beneficial for the patient. Although CCE-guided management of patients with cardiopulmonary compromise has not yet been demonstrated to improve outcome by RCTs, its unparalleled diagnostic capacity will continue to expedite recognition of life-threatening hemodynamic derangements, identify patients at high risk of increased mortality, select potential candidates for targeted therapy, quantify the effects of therapeutic changes and reduce potentially harmful interventions. As such, CCE will undoubtedly contribute to tailor therapy and improve critically ill patient outcomes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3208). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vignon P. What is new in critical care echocardiography? Crit Care 2018;22:40. [Crossref] [PubMed]
- Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 2009;135:1050-60. [Crossref] [PubMed]
- Lan P, Wang TT, Li HY, et al. Utilization of echocardiography during septic shock was associated with a decreased 28-day mortality: a propensity score-matched analysis of the MIMIC-III database. Ann Transl Med 2019;7:662. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018;44:884-92. [Crossref] [PubMed]
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. [Crossref] [PubMed]
- Papolos A, Narula J, Bavishi C, et al. U.S. Hospital Use of Echocardiography: Insights From the Nationwide Inpatient Sample. J Am Coll Cardiol 2016;67:502-11. [Crossref] [PubMed]
- Heidenreich PA, Stainback RF, Redberg RF, et al. Transesophageal echocardiography predicts mortality in critically ill patients with unexplained hypotension. J Am Coll Cardiol 1995;26:152-8. [Crossref] [PubMed]
- Blyth L, Atkinson P, Gadd K, et al. Bedside focused echocardiography as predictor of survival in cardiac arrest patients: a systematic review. Acad Emerg Med 2012;19:1119-26. [Crossref] [PubMed]
- Gaspari R, Weekes A, Adhikari S, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016;109:33-9. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Sawchuk CW, Wong DT, Kavanagh BP, et al. Transthoracic echocardiography does not improve prediction of outcome over APACHE II in medical-surgical intensive care. Can J Anaesth 2003;50:305-10. [Crossref] [PubMed]
- El-Nawawy AA, Abdelmohsen AM, Hassouna HM. Role of echocardiography in reducing shock reversal time in pediatric septic shock: a randomized controlled trial. J Pediatr (Rio J) 2018;94:31-9. [Crossref] [PubMed]
- Kanji HD, McCallum J, Sirounis D, et al. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care 2014;29:700-5. [Crossref] [PubMed]
- Ballo P, Bandini F, Capecchi I, et al. Application of 2011 American College of Cardiology Foundation/American Society of Echocardiography appropriateness use criteria in hospitalized patients referred for transthoracic echocardiography in a community setting. J Am Soc Echocardiogr 2012;25:589-98. [Crossref] [PubMed]
- Matulevicius SA, Rohatgi A, Das SR, et al. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med 2013;173:1600-7. [Crossref] [PubMed]
- Vignon P, Begot E, Mari A, et al. Hemodynamic Assessment of Patients With Septic Shock Using Transpulmonary Thermodilution and Critical Care Echocardiography: A Comparative Study. Chest 2018;153:55-64. [Crossref] [PubMed]
- Gu WJ, Wang F, Bakker J, et al. The effect of goal-directed therapy on mortality in patients with sepsis - earlier is better: a meta-analysis of randomized controlled trials. Crit Care 2014;18:570. [Crossref] [PubMed]
- Géri G, Vignon P, Aubry A, et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: a post hoc analysis. Intensive Care Med 2019;45:657-67. [Crossref] [PubMed]
- Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N Engl J Med 2016;375:1638-48. [Crossref] [PubMed]
- Lafon T, Appert A, Hadj M, et al. Comparative Early Haemodynamic Profiles in Patients Presenting to the Emergency Department With Septic and Non-Septic Acute Circulatory Failure Using Focused Echocardiography. Shock 2020;53:695-700. [Crossref] [PubMed]
- Vignon P. Continuous cardiac output assessment or serial echocardiography during septic shock resuscitation? Ann Transl Med 2020;8:797. [Crossref] [PubMed]
- Vincent JL, Rhodes A, Perel A, et al. Clinical review: Update on hemodynamic monitoring--a consensus of 16. Crit Care 2011;15:229. [Crossref] [PubMed]
- Vignon P. PRO: physician-performed ultrasound: the time has come for routine use in acute care medicine. Anesth Analg 2012;115:999-1003. [Crossref] [PubMed]
- Atkinson PR, Milne J, Diegelmann L, et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? An International Randomized Controlled Trial From the SHoC-ED Investigators. Ann Emerg Med 2018;72:478-89. [Crossref] [PubMed]


