Evaluation of the application of laparoscopy in enhanced recovery after surgery (ERAS) for gastric cancer: a Chinese multicenter analysis
Introduction
Radical surgical resection, supplemented by chemotherapy, radiotherapy or targeted therapy, is the main treatment for early and middle stage gastric cancer. For advanced or inoperable gastric cancer, some patients still can benefit from palliative chemotherapy, radiotherapy or targeted therapy. Patients who received radical surgical resection had a higher overall survival rate, however, it has many associated problems, such as a relatively large surgical field and a large number of postoperative complications. Serious complications can result in prolonged recovery time, increases medical costs, and even death (1-3). Therefore, it is important to reduce the risks of surgery and enhance patients’ recovery.
Enhanced recovery after surgery (ERAS) refers to the application of a series of management strategies evidenced to be effective in the perioperative period, which include a shortened preoperative fasting time, no indwelling nasogastric tube, and early removal of the urethral catheter. Measures such as these have been shown to alleviate the patient’s psychological and physiological stress response and reduce the postoperative complications, hospital stay time, risk for readmission, medical costs, and incidence of death (4,5). Colorectal surgery has seen the earliest and most successful application of ERAS so far, with patients discharged 2–3 days after surgery (6-8). To standardize the procedure, ERAS guidelines have been published (9). However, it remains to be seen if ERAS could be used for radical gastrectomy in the same way it has been applied with colorectal surgery. At present, many studies have reported the application of ERAS in gastric cancer and achieved satisfactory results, but of which were mostly single-center, small-sample retrospective studies with low level of evidence. Therefore, we need a multicenter, large-sample prospective clinic trial with a higher level of evidence to confirm previously reported findings. This study examined the current status of ERAS for gastric cancer by summarizing data from three medical centers in China, to prepare for a prospective clinic trial that in the hope of providing a reference value for more centers to carry out ERAS for gastric cancer and improve the implementation process of ERAS for patients with the disease.
Methods
Patients
The clinical and pathological data of 1,664 gastric cancer patients from January, 2015 to December, 2017 were collected from 3 different medical centers in China. These were Xijing Hospital of Digestive Disease, Xijing Hospital, The Air Force Military Medical University (722 patients); Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (606 patients); and Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (336 patients).
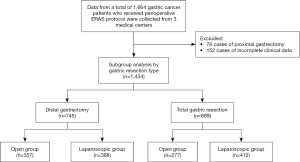
Patients diagnosed with gastric adenocarcinoma who underwent radical surgery with perioperative ERAS with American Society of Anesthesiologists (ASA) physical status classification system grades of I to III were included in the study. Patients who had received emergency surgery, or who had additional uncontrolled internal medical problems, distant metastasis detected before or during surgery, or other malignancies, were excluded. The flowchart for patient inclusion is shown in Figure 1.
This retrospective study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital and was carried out in adherence with the Declaration of Helsinki. The need for informed consent from all patients was waived due to the study’s retrospective nature.
Data collection
The patients’ basic clinical information such as age, sex, body mass index (BMI), ASA physical status classification, and tumor location were collected. Surgical and postoperative recovery information was collected including type of surgery (laparoscopic, open or laparoscopic converted to open surgery), distal gastrectomy or total gastrectomy, combined other organ dissection, operation duration, blood loss, number of lymph nodes retrieved, time until postoperative food intake, time until first flatus, time until ambulation, hospital stay duration, and complications and readmission due to complications within 30 days of discharge.
Statistical analyses
Statistical analyses were performed using SPSS 22.0 software. Quantitative data are described as the mean ± standard deviation, and t-tests or rank sum tests were used to test the hypothesis. Qualitative data are described by the number of cases and percentages, and χ2 or Fisher’s tests were used to test the hypothesis. Statistical significance was considered to exist when P<0.05.
Results
Patient clinical characteristics
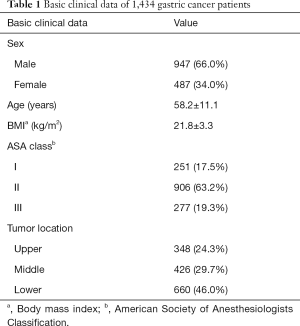
In total, 1,434 patients were involved in the final analysis, of whom there were 947 (66.0%) males and 487 (34.0%) females. The median age at diagnosis was 58.2±11.1 (range, 20–90) (Table 1).
Full table
Surgical and postoperative recovery information
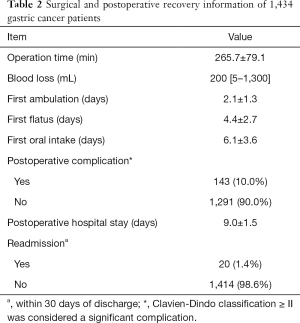
Open surgery was received by 634 (44.2%) patients and laparoscopic surgery was received by 800 (55.8%) patients, while 63 (3.3%) patients underwent laparoscopic surgery that was converted to open surgery. A total of 745 patients (52.0%) underwent distal gastrectomy, and 689 patients (48.0%) underwent total gastrectomy. The mean operation time was 265.7±79.1 min, the median blood loss was 200 [5–1,300] mL, and the number of lymph nodes dissected was 26.4±12.9. The time to ambulation was 2.1±1.3 days, time to first flatus was 4.4±2.7 days, time to first liquid food intake was 6.1±3.6 days. The postoperative length of hospital stay was 9.0±1.5 days (Table 2).
Full table
Subgroup analysis based on types of surgery and extent of resection
The types of surgery were divided into laparoscopic and open surgery (conversion of laparoscopic surgery to open surgery was considered open surgery), and the types of resection were divided into total gastrectomy and distal gastrectomy.
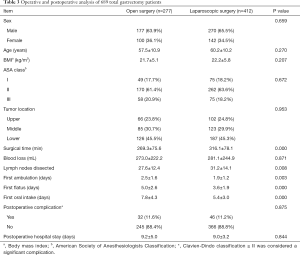
Out of the patients who underwent total gastrectomy, significantly more lymph nodes were removed in the laparoscopic group than in the open group (P<0.05). The laparoscopic group also had a shorter time to ambulation, oral intake, and first flatus, as well as a shorter postoperative hospital stay than the open group. However, the laparoscopic group had a longer operation time than the open group (P<0.05), and there were no significant differences in intraoperative blood loss or postoperative complications between the two groups (P>0.05) (Table 3).
Full table
For the patients who underwent distal gastrectomy, the laparoscopic group had less blood loss, a shorter postoperative length of hospital, and a shorter time to ambulation, oral intake, and first flatus than the open group. The operation time of the laparoscopic group was longer than that of the open group (P<0.05). There were no significant differences in the number of lymph nodes dissected or complications between the two groups (P>0.05) (Table 4).
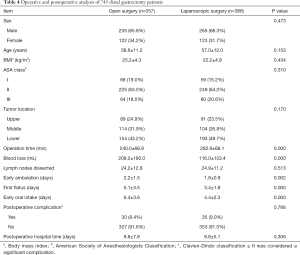
Full table
Discussion
First proposed by Danish surgeon Kehlet in 2001, the concept of fast-track surgery, or ERAS, refers to a series of management strategies for the perioperative period which were evidenced to minimize trauma for the patients and to achieve rapid recovery (5).
In China, the concept of ERAS was first introduced and promoted in 2007 by Li (10). In the same year, Li’s team first reported the safety and effectiveness of ERAS in patients with gastric cancer. Patients in the ERAS group had a shorter postoperative hospital stay, lower costs, faster postoperative exhaustion, less intravenous infusion, less weight loss after surgery, and no increase in postoperative complications, compared with the control group (11). Since then, there has been an increasing amount of research to investigate ERAS for gastric cancer in China (12,13).
However, the majority of ERAS research in China are single-center in nature and focused on a small sample size, and multicenter research with a large sample size is lacking. Our study included clinical and pathological data from 1,664 gastric cancer patients who underwent radical surgery at 3 medical centers in China, and 1,434 patients were involved in the final analysis. The purpose of this study was to determine the current status of ERAS for gastric cancer in China and to evaluate the application of laparoscopy for ERAS.
In our data, distal gastrectomy and total gastrectomy accounted for 52.0% and 48.0% of all surgeries, respectively. Only 4.7% of the patients underwent proximal gastrectomy (78/1,664 patients) due to the high risk of anastomotic leakage after proximal gastrectomy and significant reflux symptoms (14,15). Few proximal gastrectomy procedures are performed in China, Japan, and South Korea; thus, proximal gastrectomy cases were not included in this study.
Minimally invasive techniques comprise one of the core elements of ERAS. Laparoscopic surgery has been proved to result in less surgical trauma, faster recovery of postoperative gastrointestinal function, fewer complications, and shorter hospital stays. Laparoscopic methods have been used in many fields of surgery worldwide. Since the first laparoscopic distal gastric surgery for the treatment of early gastric cancer was reported by Kitano et al. in 1994 (16), laparoscopic surgery has also been carried out for total gastric cancer, achieving positive outcomes (17). In this study, in line with other research reports, 55.8% of the patients underwent laparoscopic surgery, and 3.3% of the patients underwent laparotomy that was converted from laparoscopy (17,18). These conversions have been reported to be related to the rapid development and promotion of laparoscopic surgery and reflect that the medical centers engaged in this study all had to overcome the laparoscopic learning curve.
In the subgroup analysis of patients who did and did not undergo laparoscopic surgery for total gastrectomy, the operation time for laparoscopic surgery was longer than that for open surgery (269.3±75.6 vs. 316.1±78.1 min). The difference was statistically significant (P<0.05), which was consistent with other research (17). In terms of lymph node dissection, the number of lymph nodes dissected in the laparoscopic group was greater than that in the open group (27.6±12.4 vs. 31.2±14.1, P<0.05), which could be attributed to the better surgical field offered by laparoscopic surgery. There was no significant difference in intraoperative blood loss or postoperative complications (P>0.05) between the two groups. The time to first ambulation, oral intake, and first flatus were all significantly shorter for the laparoscopic group than for the open group (P<0.05).
In those who underwent distal gastrectomy, the laparoscopic group had significantly less intraoperative blood loss, and earlier ambulation, oral intake and first flatus, as well as a shorter postoperative hospital length of stay than the open group (P<0.05). The operation time for laparoscopic surgery was also longer than that for open surgery (P<0.05). There was no difference in the number of lymph nodes dissected or postoperative complications (P>0.05) between the two groups.
In this study, the laparoscopic distal gastrectomy group experienced less blood loss than the open group (P<0.05), but there was no significant difference between the laparoscopic and open total gastrectomy groups (P>0.05). This is most likely due to the longer learning time for laparoscopic total gastrectomy than for partial gastrectomy (19,20). Therefore, the results of this subgroup study suggest that compared to open surgery, other having a longer operation time, radical laparoscopic gastrectomy can retrieve a similar number of lymph nodes and also promote postoperative recovery without increasing the rate of postoperative complications.
Postoperative complications are indicative of the quality of the surgery. Due to the limitations of retrospective analysis, our study only included complications classified as higher than grade II by the Clavien-Dindo scoring system. In total, 143 patients (10.0%) had postoperative complications. In the total gastrectomy group, the postoperative complication rates were 11.6% and 11.2% in the laparoscopic and open groups, respectively. In the distal gastrectomy group, the laparoscopic group had an 8.4% postoperative complication rate, while the open group had a 9.0% postoperative complication rate. No significant differences were shown in either subgroup analysis, which is consistent with the result of the CLASS01 trial (18).
A meta-analysis that included 8 studies in gastrectomy for gastric cancer and compared a total of 399 ERAS patients with 402 normal patients showed that the ERAS patients had shorter postoperative hospital stays and lower inpatient costs but a higher rate of readmission than the normal patients, and there were no differences observed in operation time, number of lymph nodes dissected, catheter indwelling time or postoperative complication rate (21). In our study, the time to first ambulation, oral intake, and first flatus were all shorter in the laparoscopic group than in the open surgery group, and the laparoscopic group had a shorter hospital stay than the open surgery group. However, due to regional economic differences, medical costs were not included in this analysis.
Despite being a relatively new concept in perioperative management, ERAS already has guidelines for its application in many different fields, such as colorectal, gastric, gynecologic, and cardiothoracic surgery. To date, however, there are no standardized criteria for evaluating ERAS management. A recent Spanish prospective, multicenter cohort study (the POWER study), involving 2,084 patients who underwent colorectal surgery (22), found that the general adherence rate to ERAS was 63.6% (IQR, 54.5–77.3%), while the adherence rate of the non-ERAS group was 59.1%, which was a statistically significant difference (P<0.001). The increased rate of compliance to ERAS was most likely due to the reduction in postoperative complications. Our study group will report the influence of postoperative complications on the completion rate of ERAS in future research.
Our study has certain limitations. Firstly, due to its retrospective nature, some data were incomplete. Secondly, in the early stages of ERAS implementation, patients in good condition may be assigned to ERAS group which lead to bias of the results. Thirdly, due to the uneven development of the region, we can’t analyze the health-economic indicators such as cost of hospitalization.
Although our study simply described the current status of ERAS for gastric cancer in three medical centers in China, our further plan is to perform a prospective clinic trial providing a higher level of evidence for more centers to carry out ERAS for gastric cancer.
Conclusions
In conclusion, ERAS is safe and effective for radical gastrectomy for gastric cancer. Laparoscopic gastrectomy combined with ERAS can shorten the time to early ambulation, oral intake, and first flatus and can also shorten the postoperative length of hospital stay, without compromising oncological outcome or increasing the incidence of postoperative complications. However, further investigation of the long-term outcomes with a larger-scale, prospective, multicenter, randomized controlled trial is still required.
Acknowledgments
Funding: JW received personal grants from the Ethicon Excellence in Surgery Grant (EESG) (No. HZB-20181119-49) and Science and Technology Program Guangzhou, (No. 201904010020), China.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2556). JW reports grants from the Ethicon Excellence in Surgery Grant (EESG), outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital and was carried out in adherence with the Declaration of Helsinki [No. GDREC2019296H(R1)]. The need for informed consent from all patients was waived due to the study’s retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Solanki S, Chakinala RC, Haq KF, et al. Inpatient burden of gastric cancer in the United States. Ann Transl Med 2019;7:772. [Crossref] [PubMed]
- Watanabe M, Miyata H, Gotoh M, et al. Total gastrectomy risk model: data from 20,011 Japanese patients in a nationwide internet-based database. Ann Surg 2014;260:1034-9. [Crossref] [PubMed]
- Wang Y, Li J, Weng Y, et al. A new enhanced recovery after surgery pathway for left-sided pancreatic cancer patients after distal pancreatectomy. Transl Cancer Res 2019;8:2613-20. [Crossref]
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322:473-6. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763-4. [Crossref] [PubMed]
- Kehlet H. Fast-track colorectal surgery. Lancet 2008;371:791-3. [Crossref] [PubMed]
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014;101:1209-29. [Crossref] [PubMed]
- Jiang Z, Li J. Current Status of Enhanced Recovery After Surgery in China. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:246-9. [PubMed]
- Liu XX, Pan HF, Jiang ZW, et al. "Fast-track" and "Minimally Invasive" Surgery for Gastric Cancer. Chin Med J (Engl) 2016;129:2294-300. [Crossref] [PubMed]
- Feng F, Ji G, Li JP, et al. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 2013;19:3642-8. [Crossref] [PubMed]
- Chen Hu J, Xin Jiang L, Cai L, et al. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1830-9. [Crossref] [PubMed]
- Piessen G, Triboulet JP, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg 2010;147:e273-83. [Crossref] [PubMed]
- Chin AC, Espat NJ. Total gastrectomy: options for the restoration of gastrointestinal continuity. Lancet Oncol 2003;4:271-6. [Crossref] [PubMed]
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Hyung WJ, Yang HK, Han SU, et al. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer 2019;22:214-22. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- Jeong O, Ryu SY, Choi WY, et al. Risk factors and learning curve associated with postoperative morbidity of laparoscopic total gastrectomy for gastric carcinoma. Ann Surg Oncol 2014;21:2994-3001. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Monma E, et al. Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 2015;29:1321-6. [Crossref] [PubMed]
- Ding J, Sun B, Song P, et al. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017;8:75699-711. [PubMed]
- Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery: The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg 2019;154:725-36. [Crossref] [PubMed]