Do metformin a real anticarcinogen? A critical reappraisal of experimental data
Introduction
In a history of each outstanding drug like a history of each distinguished person there are a number of particular points determined their fate, achievements and future. As it was elegantly described in some works (1-4), in the early 1900s, guanidine was identified as active compound of botanic medicine Galega officinalis (French Lilac) commonly used in medieval Europe for the treatment of polyurea in diabetic patients. This was followed by development of antidiabetic biguanides in the 1920s, however due to the discovery of insulin in 1921 only 30 years late the first biguanides 1-phenylethylbiguanide [phenformin (PF)], 1-butylbiguanide hydrochloride [buformin (BF)] and N,N-dimethylbiguanide [metformin (MF)] were synthesized. The drugs were approved in 1957 in the USA and Europe for the treatment of diabetes mellitus type 2.
In the 1971, Dilman (5) created the idea that the antidiabetic biguanides may be promising as potent anti-aging and anti-cancer drugs. In series of experiments in mice and rats started in the middle of 1970s at the N.N. Petrov Research Institute of Oncology, it was shown that antidiabetic biguanides PF and BF given soon after puberty increased the life span and postponed or suppressed the development of spontaneous tumors and/or inhibits carcinogenesis induced by different chemicals or X-ray radiation in a number of localization (1,6-18). It was shown also that PF potentiated antitumor effect of cytostatics in mice and rats (19,20). Dilman (5) suggested also that age-related increase in hypothalamic threshold of sensitivity to homeostatic inhibition by estrogens is a key mechanism of aging of the reproductive system. In our study, daily treatment of hemicastrated young rats with 0.57 µg of diethylstilbestrol inhibited compensatory ovarian hypertrophy (COH) by 48% in the 3-month animals and by 3% only in the 18-month animals. Administration of the same dose of estrogen to old rats simultaneously given PF suppressed the COH by 65-98% (21,22). These data suggest a functional nature of the age changes in the hypothalamic sensitivity to the estrogen action. Much later it was shown significant effect of biguanides as inducer of ovulation in polycystic ovarian syndrome (23,24).
Dilman proposed metabolic rehabilitation for cancer patients giving them with biguanides and maintained on calorie restricted diet (1,25-28). Treated breast, stomach and colon cancers patients had reduced incidence of metastases, and less primary tumor development. In the late 1979s due to high incidence of adverse effects (severe lactic acidosis) PF was deleted from clinical use and MF possessed in much less adverse effects started toward nowadays (1-4). In 2005 we have found that treatment with MF inhibits mammary carcinogenesis and increases the life span of female transgenic HER-2/neu mice (29). At the same year, Evans et al. (30) and then Bowker et al. (31) reported that treatment with MF decreased the breast cancer risk. These studies induced an exponential growth of publications on MF and cancer. A search in the PubMed on “metformin and cancer” shows no publication in 1991, 33 papers in 2001, 88 in 2005, 175 in 2010, 223 in 2011, and 313 in 2013. From 1st January to 3rd March 65 papers on the topic appeared in PubMed! There are a near hundred excellent reviews and meta-analyzes of epidemiological data on MF effect on cancer risk in type 2 diabetes patients. We refer readers to some of them (1-4,32-34). Two years ago, in a comment “Cancer prevention with a diabetic pill?” published on in Science (35) it was stressed that “diabetics treated with MF have from 25% to 40% less cancer than those who receive insulin as therapy or take sulfonylurea drugs that increase insulin secretion from the pancreas. MF already may have saved more people from cancer death than any drug in history. Some 120 million prescriptions are written for it yearly.”.
The aim of this paper to review data received in laboratory animals in vivo in studies focused not on the treatment but on the prevention of cancer development. There is considerable evidence for anti-tumor effects of antidiabetic biguanides both in vitro and in vivo. In this paper, we shall not discuss this aspect of the biguanides action. This paper focuses mainly on data received in laboratory animals in vivo in studies on the capacity of the biguanides to prevent spontaneous and induced tumorigenesis. The evaluation of effects of biguanides on life span and longevity of animals was done elsewhere (36-42).
Effect of antidiabetic biguanides on total tumor development in rodents
The data on effect of antidiabetic biguanides on spontaneous and induced by various chemical, physical and biological carcinogenic agents are summarized in the Table 1.
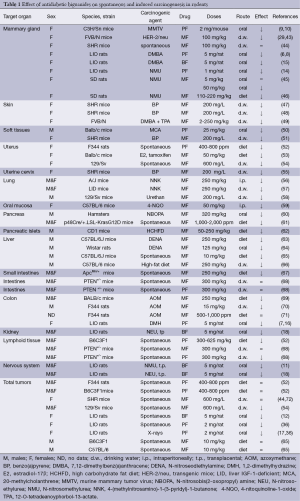
Full table
In the National Cancer Institute Bioasay of Phenformin for Possible Carcinogenicity (52), F344 rats and B6C3F1 mice of both sexes were used. The matched control groups included 15 animals each whereas groups exposed to PF consists 35 animals each. PF was given in low and high doses (400 and 800 ppm) in diet during 78 weeks starting at the age of 8 weeks both to rats and mice. The dosages for mice and rats calculate from food consumption were 300-625 mg/kg/day. The treatment was followed by observation period of 26 weeks, than all survived animals were sacrificed. The average body weights of both high- and low-dose female F344 rats were consistently lower than the controls during the treatment period, while body weights of the males were essentially unaffected by the drug. Until the age of 105 weeks survived 53% of control male rats, 67% of high-dose and 91% low-dose treated males. In the female rats, there were no significant differences between the mortality of the different groups. 83% of the high-dose group survived to the end of the study, 67% in control and 68% in low-dose group. The incidences of tumors of any localization in males as well as in the majority of tumors in female rats were similar in the controls and in PF treated groups. However, incidences of tumors of reproductive system were statistically lower (21% and 17%) than that in the matched controls (47%) (P<0.027). The mean body weights of both male and female B6C3F1 mice were markedly lower than controls during the first 60-78 weeks of administration of PF at the low- and high doses. Until the age of 105 weeks survived 13% controls, 19% low-dose and 29% high-dose treated male mice and 33%, 59% and 52% of female mice, correspondingly. The incidence of hematopoietic tumors was 33% of the matched controls of both male and female mice, compared to only 1.5% hematopoietic tumors in the male and 5.4% of the female lab historical controls. The conclusion of the bioassay was that there is no evidence that under the conditions of this bioassay PF was carcinogenic. It worthy to note that in male F344 rats and female B6C3F1 mice rather increase of the survival was registered in PF treated groups. The treatment with the drug decreased also the incidence of reproductive system tumors in female rats and lymphomas in male and female mice. Unfortunately the all survived until the age of 112 weeks animals were sacrificed. Thus, this study does not allow animals to survive until natural death and to evaluate geroprotective effect of PF.
In our study (36) PF was given orally five times a week to 44 female outbred LIO rats starting from the age of 3.5 months until a natural death in a single dose of 2 mg/rat/day. Forty one orally treated with tap water rats served as control. Administration of PF failed to influence the mean life span of rats. At the same time, the mean life span of the last 10% survivors was increased by 10% (P<0.01), and maximum life span was increased by 3 months (+10%) in comparison with the controls. The treatment with PF slightly decreased the body weight of rats and postponed the age-related switching-off estrous function as compared with the control group. Spontaneous tumor incidence was decreased by 1.6 times in PF-treated rats, however the incidence of fatal tumors was similar to controls.
Forty three female LIO rats were given per os with intragastral instillation BF five times a week at a single dose of 5 mg/rat (18-25 mg/kg of b.w.) starting at the age of 3.5 months until natural death. Seventy five control rats were given a tap water. BF decreased body weight of rats from the 12nd to 20th month of life and slowdown age-related switching-off of estrous function as compared to the control. BF increased the by 7.3% the mean life span and by 12% the mean life span of last 10% survivors. Total tumor incidence was 30.7% in controls and 18.6% in BF treated rats (P<005), the tumor multiplicity and (number of tumors per tumor-bearing animal) was 0.40 and 0.23, respectively. There was no significant difference in the incidence of specific tumors between the control an BF-treated rats. The mean life span of tumor-bearing rats exposed to BF was increased 7.3% as compare to the controls (12).
Outbred Swiss-derived 3-month-old female SHR mice were randomly divided into two groups. Mice of the first group were given MF with drinking water (600 ppm =100-150 mg/kg) daily, whereas mice of the second group were given tap water without MF and served as a control. There was no difference in the mean body weight of mice exposed and non-exposed to the drug until the age of 20 months and a tendency to a decrease of the body weight was observed after this age in MF-treated group. MF postponed the age-related disturbances in estrous function in mice and by 37.9% increased the mean life span of mice. The incidence of spontaneous tumors in MF-treated and non-treated group was 65.2% and 55%, respectively. MF failed influence the incidence of any type of malignancies in these mice (44).
In another experiment we studied the role of the age at start of MF treatment on life span and spontaneous tumorigenesis in female SHR mice (72). MF (600 ppm =100 mg/kg) was given with drinking water starting from the age of 3, 9 or 15 months. According to the estimated parameters of the Cox’s regression, MF administration from the age of 3 months decreased the relative risk of death compared to the control group, whereas MF treatment started at the age of 9 or 15 months produced no effect on the risk of death in SHR mice. Total tumor incidence was practically similar in all MF-treated and non-treated groups. There were no cases of lung adenocarcinomas in the mice treated with MF from the age of 3 or 15 months whereas three cases of this malignancy were detected in the relevant controls.
Inbred female 129/Sv mice were given MF (600 ppm =100 mg/kg) with drinking water or only tap water without a drug starting from the age of 3 months until natural death (54). MF slightly increased mean life span of 129/Sv mice. However, it is worthy to note, that until the age of 700 days survived 54.1% if mice of the control group and 72.9% of MF-treated females (P<0.03). The total tumor incidence was similar in the control and MF-treated group. However, the incidence of malignant tumors was significantly decreased (by 3.5 times) in the group given MF in comparison to the control.
In recent paper by Martin-Montalvo et al. (65) male C57BL/6 mice were given ad libitum diet with supplementation of 0.1% (1,000 ppm) or 1% (10,000 ppm) of MF starting from the age 54 weeks for the remainder of their lives. The mean life span of mice treated with 0.1% in diet was increased by 5.83% as compared with the relevant control group of mice, whereas the dose 1% was toxic and reduced the mean life span by 14.4%. Diet supplementation with 0.1% MF increased lifespan by 4.15% in another strain of mice, B6C3F1. No numerical data on maximal lifespan of mice of any group were presented in the paper. It was observed that C57BL/6 mice were lighter than those in control group between the age of 72 to 90 weeks and were heavier them by the age of 124 weeks. These effects were not observed in male B6C3F1 mice. There were no significant differences in pathologies observed in both strains of mice fed diet with 0.1% MF. However, as it could be calculated from the data presented by these authors in their supplementary table, diet with 1% MF led to significant reduction of liver cancers incidence (3.3% in the MF group and 26.5% in control group, P<0.001). Male C57BL/6 mice given MF have lower rates of cataracts. The authors stressed that treatment with MF mimics some of the benefits of calorie restriction, such as improved physical performance, prevented the onset of metabolic syndrome: improved glucose-tolerance test, increased insulin sensitivity, and reduced low-density lipoprotein and cholesterol levels without a decrease in caloric intake. At a molecular level, MF increased, MF increases AMP-activated protein kinase activity and increases antioxidant protection, resulting in reductions in both oxidative damage accumulation and chronic inflammation (65). MF also exerts CR-like genomic and metabolic responses which were interpreted as induction of associated with longevity pathways in mice.
Mammary carcinogenesis
In 1974, Dil’man et al. (6) were the first who reported the inhibitory effect of PF on chemically induced mammary carcinogenesis in rats. Female outbred rats were subjected to 4 intravenous injection of 7,12-dimethylbenz(a)anthracene (DMBA) at a single dose of 2 mg per rat with a week intervals. Starting at the first day of the carcinogen treatment a part of rats were given orally with PF at a single dose of 5 mg/rat (approximately 30-45 mg/kg of the body weight) during 2.5 months. The experiment was terminated at the 5th month after the first DMBA injection. The first mammary tumors were detected in 2 months after start in the control group (DMBA only) and 3 weeks later in DMBA + PF group. Mammary tumors developed in 100% control rats and only in 43% of PF-treated animals (P<0.01). The multiplicity and size of tumors were also decreased in PF group. It is worthy to note that low-differentiated mammary carcinomas were detected only in rats exposed to DMBA alone, and never—in DMBA + PF group. Using the same model of mammary carcinogenesis in another set of experiments daily dose of PF was increased to 10 mg/rat but the treatment was started on 5th day after the first injection of the carcinogen. Results were similar to previous one (8).
Long-term treatment with PF (2 mg/mouse/day =80-100 mg/kg, orally with intragastral instillation) significantly inhibited (by 4.0-fold, P<0.01) the incidence and multiplicity (number of tumors per tumor-bearing animal) of spontaneous mammary adenocarcinomas in female C3H/Sn mice infected with murine mammary tumor virus (9,10). The tumor yield curve was significantly shifted to right as a result of the treatment. MF (600 ppm) given with drinking water inhibited mammary carcinogenesis in HER-2/neu transgenic mice (29,43), but it had no effect on incidence or latency of mammary adenocarcinomas in outbred SHR mice (44). PF-treated rats tended to have lower serum insulin levels. Treatment with PF normalized glucose tolerance, serum insulin and IGF-1 level in rats exposed to intravenous injections of N-nitrosomethylurea (NMU). It also inhibited mammary carcinogenesis in these animals (14).
Bojkova et al. (45) studied oral MF’s chemopreventive effect on mammary carcinogenesis in female Sprague-Dawley rats. NMU administered in two intraperitoneal doses of 50 mg/kg b.w. between the 43rd-55th postnatal days induced mammary carcinogenesis. MF was administered in drinking water (at a concentration of 5 and 50 mg/L, corresponding to 5 and 50 mg/kg) 13 days before the first NMU dose until the termination of the experiment. The experiment was terminated 18 weeks after the first NMU injection. MF did not significantly alter the tumor growth, although a delay in tumor onset was recorded after a higher MF dose. Tumor incidence was decrease at the 8th week and tumor latency was increased until the 12th week in MF50 group in comparison with MF5 rats. Tumor growth parameters were not significantly changed by treatment with MF, however MF altered metabolic and hormonal variables. Insulinemia was decreased after either dose of MF in comparison with control rats without changes in glycemia. Triacylglycerols concentration decreased in liver and increased in serum when compared to controls. Moreover, the higher dose of MF attenuated lipid peroxidation in the liver.
In study by Zhu et al. (46), 21-day-old female Spargue-Dawley rats were injected intraperitoneally with a single dose 50 mg/kg NMU. Six days following carcinogen injection, 90 rats were subdivided into three groups: diet without MF, and diet with MF 0.5% or 1.0% w/w (~110 or 220 mg/kg) for 5 days, and then MF 0.05% or 0.25% w/w (11 or 55 mg/kg) until 28th day. In this experiment MF 0.5%/0.05% decreased cancer incidence and reduced tumor multiplicity and burden (tumor mass per rat) and increased its latency. MF 1.0%/0.25% significantly reduced mammary carcinomas multiplicity and burden. It worthy of note that smaller dose of MF (0.3% w/w) given at early stage of carcinogenesis was ineffective in mammary carcinogenesis inhibition. The finding suggests that early events in the carcinogenic process are more susceptible to high dose of MF (46). While a dosing regimen of 1.0%/0.25% MF reduced palpable mammary carcinomas incidence, multiplicity, and tumor burden and prolonged latency, lower doses of MF failed to inhibit carcinogenesis despite effects on plasma insulin. In human breast cancer, cell growth inhibition was observed only at high concentrations of MF. Poor in vivo and in vitro response to MF may be the result of pharmacokinetic (OCT-1 expression was low in rat mammary cells; OCT-3 was downregulated in mammary carcinoma) and pharmacodynamic (complex I transcripts were higher in mammary epithelial cells from carcinomas versus uninvolved gland) effects. Combined with dietary energy restriction, MF protected against new tumor occurrence after release from combined treatment. Flow cytometry indicated the presence of cancer-initiated cells in mammary carcinomas. The authors concluded that, as a single agent, MF possessed limited cancer inhibitory abilities. However, MF may be an effective component of multi-agent interventions that target cancer-initiated cells. There is a clear need to identify the conditions under which MF is likely to prevent and control breast cancer.
Skin carcinogenesis
In the experiments of Deriabina et al. (47), one hundred and twenty SHR female mice were randomly divided into four groups. Their clean-shaven backs were painted with 0.2 mL of 0.05% acetone solution of BP twice a week. Group I was in control and received no additional treatment. From beginning to end (6 months), the remaining mice received melatonin 2 mg/L with drinking water at nighttime (group II), MF 200 mg/L (200 ppm) with drinking water during 24 hrs (group III), melatonin and MF as in groups II,III (group IV). There was a separate group of intact animals. Skin tumor frequency decreased among melatonin/MF-treated mice (groups I-IV)—83.3%, 66.7%, 60% and 50%; squamous cell carcinoma—56.7%, 36.7%, 20% and 20%. Treatment with melatonin and MF and their combinations was followed by significantly lower tumor multiplicity and smaller size, longer latency period and survival of tumor-bearers. After BP, levels of malonic dialdehyde (MDA) and catalase rose in blood serum while concentrations of the latter in skin tumors were higher than in cutaneous homogenates in intact animals. Melatonin and MF significantly lowered MDA content of blood serum as compared with group I. Blood serum catalase fell after their joint administration, whereas it was 2.6 times as high in cutaneous homogenates after MF. However, it decreased after melatonin (47).
Female SHR mice (n=200) were divided at random into four groups, 50 per group (48) 0.2 mL of 0.05 BP solution in acetone was applied to a skin site on the back for 26 weeks. In parallel, the animals received melatonin, MF or both. Treatment with melatonin and MF and their combinations was followed by significantly lower tumor multiplicity (number of tumors per tumor-bearing animal) and smaller size, longer latency period and survival of tumor-bearers. After BP, levels of MDA and catalase increased in blood serum while concentrations of the latter in skin tumors were higher than in cutaneous homogenates in intact animals. Melatonin and MF significantly lowered MDA content of blood serum as compared with group I. Blood serum catalase fell after their joint administration, whereas it was 2.6 times as high in cutaneous homogenates after MF. However, it decreased after melatonin (48). The ability of MF to inhibit skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate (TPA) was analyzed in mice maintained on either an overweight control diet or an obesity-inducing diet (49). Rapamycin was given topically for comparison, and a combination of MF and rapamycin was studied as well. MF (given in the drinking water) and rapamycin suppressed both papillomas and squamous cell carcinomas in overweight and obese mice in a dose-dependent manner. A low-dose (20 ppm) combination of these two compounds displayed an additive inhibitory effect on tumor development. MF treatment also reduced the size of papillomas. It is worthy to note, all treatments seemed to be at least as effective for inhibiting tumor formation in obese mice, and both MF and rapamycin were more effective at reducing tumor size in obese mice compared with overweight control mice. The effect of MF on skin tumorigenesis was associated with a significant reduction in TPA-induced epidermal hyperproliferation. Furthermore, treatment with MF led to activation of epidermal AMP-activated protein kinase (AMPK) and attenuated signaling through mammalian target of rapamycin complex (mTORC)-1 and p70S6K. Combinations of MF and rapamycin were more effective at blocking epidermal mTORC1 signaling induced by TPA consistent with the greater inhibitory effect on skin tumor promotion. Thus, MF effectively inhibits skin carcinogenesis.
Soft-tissue carcinogenesis
Vinnitski and Iakumenko (50) have studied effect of treatment with PF on carcinogenesis induced by a single subcutaneous administration of 0.1 mg 20-methylcholanthrene (MCA) in male BALB/c mice. Three curses of treatment with PF in a single dose 25 mg/kg by intragastric instillation during 5 days alone or with combination with 5 daily subcutaneous injection of live lyophilized vaccine BCG (0.1 mg/kg) or yeast polysaccharide zymozan (20 mg/kg) was given with a week intervals. Three months after MCA administration subcutaneous sarcomas developed in 73% mice of the control group, in 61%, 88% and 78% of mice treated with PF, BCG or zymozan alone and in 50% and 38% mice treated with PH + BCG or PF + zymozan, respectively. Four and half months after the carcinogen injection sarcomas were detected in 100% mice of the control group, in 89%, 100% and 94% of animals exposed to PF or to immunostimulators alone, and in 72% and 67% mice treated with PF + BCG or PF + zymozan, correspondingly.
In the experiments of Deriabina (51), 120 of 2-month-old female SHR mice were randomly divided into four groups. All animals were subjected to a single, subcutaneous injection of 2 mg of BP. Group I was in control and received no additional treatment. Starting form the next after carcinogen treatment during 26 weeks mice were given with drinking water melatonin 2 mg/L during the night time (group II), MF 200 mg/L (260-320 mg/kg) with drinking water during 24 hrs (group III), and melatonin and MF as in groups II,III (group IV). There was also additional group of intact animals. Subcutaneous malignant fibrous histiocytomas developed in 80%, 60%, 46.7% and 40% of mice in groups I-IV, correspondingly. MF and melatonin, as well as its combination, increased survival of tumor-bearing mice. The author observed significant inhibitory effect of MF on mitotic activity in tumor tissue (51). Thus, in both models antidiabetic biguanides inhibits tumorigenesis in soft tissues of mice.
Uterine carcinogenesis
Forty-eight oophorectomized Balb/c mice were randomly assigned to receive saline, tamoxifen citrate (4 mg/kg), 17β-estradiol hemihydrate (4 mg/kg), MF (50 mg/kg), tamoxifen citrate (4 mg/kg) with MF (50 mg/kg) or estradiol (4 mg/kg) with MF (50 mg/kg) for 3 days. Histological markers of uterotrophy, including luminal epithelial cell height and density of endometrial glands, were quantified. Immunohistochemical expression of PCNA and S6K1 was evaluated. H-score was used for S6K1 expression (53). Mice treated either with tamoxifen or estradiol had significantly increased density of endometrial glands and epithelial height compared to vehicle-only or MF-only group (P<0.001). Addition of MF to tamoxifen or estradiol treatment significantly decreased the density of endometrial glands and epithelial cell height (P<0.05). Addition of MF to tamoxifen significantly decreased the H-score of S6K1 (P<0.05) and the immunohistochemical expression of PCNA (P<0.05) in uterine lining epithelium, glandular and stromal cells. Addition of MF to estradiol significantly decreased the H-score of S6K1 (P<0.05) and the immunohistochemical expression of PCNA (P<0.05) in uterine-lining epithelium, glandular and stromal cells. The authors suggest that MF appears to have antiproliferative effects on the endometrium of estradiol or tamoxifen-treated mice via inhibiting the mTOR-mediated S6K1 activation.
It worthy to note that treatment with MF attenuated estrogen-dependent proliferative expression of c-myc and c-fos in Zucker fa/fa obese rat endometrium compared to untreated controls and was accompanied by inhibition of phosphorylation of the insulin and IGF1 receptors (IRβ/IGF1R) and ERK1/2 (73). The authors showed in in vitro studies that MF inhibited RENE1 proliferation in a dose-dependent manner.
Treatment with MF reduced the incidence (by 23%) and size (by three times) and increased (by 35%) the latency of cervicovaginal squamous-cell carcinomas induced by BP intravaginal applications in SHR mice (55). MF significantly reduced mitotic activity, number of CD31+ positive vessels and VEGF staining in these tumors. Benign vascular tumors of the uterus and ovarian haemangiomas developed most frequently in MF-exposed female 129/Sv mice. Thus, in toto uterine and ovarian haemangiomas and hemangioendotheliomas were revealed respectively in 54.2% of control and 68.8% of MF-exposed female mice, P<0.03 (Fisher’s exact test). At the same time, the differences in the incidence of angiogenic tumors only of ovaries or uterus between treated and untreated with MF was not statistically significant (54).
Hepatocarcinogenesis
In the study by Bhalla et al. (63) 2-week-old male C57BL/6J mice were injected intraperitoneally with 25 mg/kg body weigth diethylnitrosamine (DEN). Mice were weaned at 4 weeks of age and fed control chow or chow containing MF at a dose of 250 mg/kg body weight for 24 or 36 weeks. MF-treated mice developed 57% fewer tumors compared with control chow-fed mice when evaluated at the 24th week. The size of liver tumors was also reduced approximately 37% in MF-treated mice compared with control ones. In 36-week group, MF treatment reduced the number of liver tumors almost 60%. The size of tumors was also reduced significantly. The authors observed that MFc reduces blood glucose levels in DEN-treated mice without inducing clinical hypoglycemia. Bhalla et al. (63) show in these studies that MF protected mice against chemically induced liver tumors. Interestingly, MF did not increase activation of AMPK, a known target of MF. Rather, MF decreased the expression of several lipogenic enzymes and lipogenesis. In addition, restoring lipogenic gene expression by ectopic expression of a transcription factor SREBP1c rescues MF-mediated growth inhibition. This mechanism of action suggests that MF may also be useful for patients suffering from other disorders associated with HCC in which lipid synthesis is increased. As a whole, these studies show that MF prevents HCC and that it should be evaluated as a preventive agent for HCC in readily identifiable, at-risk patients.
Afzal et al. (64) studied effect of a single dose of 200 mg/kg MF on DENA–induced hepatocarcinogenesis in male Wistar rats and observed inhibition of hepatocarcinogenesis which has been monitored by estimation of α-feto protein (AFP). The level of AFP in the serum of rats in vehicle control group was 22 mg/dL, the treatment with MF (125 mg/kg) during 16 weeks did not changes this value and was 19 mg/dL. A single dose of DENA was followed by 297 mg/dL of AFP. MF was given from day 1 and after 7th day the carcinogen was administered or rats were treated with DENA and after 7th day MF treatment was initiated. The levels of AFP in these groups were 48 and 64 mg/dL, respectively.
Tajima et al. (66) have found that MF prevented high-fat diet induced liver carcinogenesis in C57BL/6 mice. It is worthy to note that metformin failed to inhibit tumors when the treatment was started in mice with developed liver disease.
Pancreatic carcinogenesis
Schneider et al. (60) examined whether the prevention of islet cell proliferation can inhibit the promotional effect of a high-fat diet in carcinogenesis. Two groups of high-fat-fed hamsters were used. One group received MF in drinking water for life (HF + MF group), and the other group served as a control (HF group). At the time when the normalization of the plasma insulin level was expected, all hamsters were treated with the pancreatic carcinogen, N-nitrosobis-(2-oxopropyl)amine. The experiment was terminated 42 weeks later. Although 50% of the hamsters in the high-fat group developed malignant lesions, none was found in the HF + MF group (P<0.05). Also, significantly more hyperplastic and premalignant lesions, most of which were found within the islets, were detected in the high-fat group (8.6 lesions/hamster) than in the HF + Met group (1.8 lesions/hamster). The authors stressed that the results lend further support to islet cells’ significant role in pancreatic carcinogenesis and may explain the association between pancreatic cancer and obesity, which is usually associated with peripheral insulin resistance.
CD1 mice were randomized into five groups, receiving a high-carbohydrate, high-fat (HC-HF) hyperinsulinemia-inducing diet with 50, 100 or 250 mg/kg body weight (mg/kg) MF in drinking water, a standard diet or HC-HF diet alone (62). Animals on the HC-HF diet developed obesity and insulin resistance. They had significantly higher body weight, upper-normal fasting blood glucose, higher insulin secretion and utilization, and fatty degeneration of the liver. MF at the doses employed significantly reduced food and water consumption; however, only a dose of 250 mg/kg significantly reduced body weight gain and suppressed gluconogenesis and produced a remarkable reduction in insulin secretion. There was no observed MF-related hepatotoxicity in any of the groups. In summary, MF at various doses exhibits protective effects on the metabolic disorder caused by the HC-HF diet, with the most effective protection at a dose of 250 mg/kg. These effects may explain its translational role relating to its anti-neoplastic potential in the human (62).
Muhammed et al. (61) evaluated the effects of MF on pancreatic intraepithelial neoplasia (PanIN) and their progression to pancreatic ductal adenocarcinoma (PDAC) in p48Cre/+.LSL-KrasG12D/+ transgenic mice. Mice fed control diet showed 80% and 62% incidence of PDAC in males and females, respectively. Male mice showed 20% and 26%, and female mice showed 7% and 0% PDAC incidence with 1,000- and 2,000-ppm MF treatments, respectively. Both doses of MF decreased pancreatic tumor weights by 34% to 49% (P<0.03-0.001). The drug treatment caused suppression of PanIN 3 (carcinoma in situ) lesions by 28% to 39% (P<0.002) and significant inhibition of carcinoma spread in the pancreas. The pancreatic tissue and/or serum of mice fed MF showed a significant inhibition of mTOR, extracellular signal-regulated kinases (ERK), phosphorylated extracellular signal-regulated kinases (pErk), and insulin-like growth factor 1 (IGF-1) with an increase in phosphorylated 5’ adenosine monophosphate kinase (pAMPK), tuberous sclerosis complex 1 (TSC1, TSC2), C-protein and an autophagy related protein 2 (ATG2). The cancer stem cell (CSC) markers were significantly decreased (P<0.04-0.0002) in the pancreatic tissue. These results suggest that biologic effects of MF are mediated through decreased CSC markers cluster of differentiation 44 (CD44 and CD133), aldehyde dehydrogenase isoform 1 (ALDH1), and epithelial cell adhesion molecule (EPCAM) and modulation of the mTOR signaling pathway. These data shows that MF has significant potential for use in clinical trials for PC chemoprevention.
Digestive tract carcinogenesis
Vitale-Cross et al. (59) observed that MF prevented the development of oral cavity squamous cell carcinoma (HNSCC) induced with 4-NQO by significantly reducing the size and number of carcinogen-induced oral tumoral lesions and by preventing their spontaneous conversion to squamous cell carcinomas. Female C57BL/6 mice were treated with 4-NQO during 14 weeks and then subdivided into two groups—control [injected intraperitoneally (i.p.) with sterile saline] and treated with MF (50 mg/kg i.p.) for 8 weeks. By the week 22 mice exposed to the carcinogen developed visible and palpable oral tumors mainly localized on the tongue. The number and size of the visible oral lesions were significantly decreased in MF-treated mice. MF treatment decreased also a number of squamous cell carcinomas to a single case among ten mice whereas control mice exhibited one to two cancers per mouse.
Subcutaneous administration of 1,2-dimethylhydrazine (DMH) into (once a week for 4 weeks) caused the decrease in the level of biogenic amines, particularly dopamine in the hypothalamus, alongside a decrease of glucose tolerance and an increase in insulin and triglyceride blood levels. The exposure to DMH also caused inhibition of lymphocyte blastogenic response to phytohemagglutinin and lipopolysaccharide; a decrease in the level of antibody produced against sheep erythrocytes; and a decrease in phagocytic activity of macrophages (7). Administration of PF started from the first injection of the carcinogen alleviated all of the above-mentioned immunological tumor—prone changes.
The number of colon adenocarcinomas per animal induced in female rats with DMH (21 mg/kg s.c. once a week for 23 weeks) was 6.61±0.71), however it was 4.80±0.60 (P<0.05) in rats treated 5 days a week with 5 mg of PF (~35-40 mg/kg) starting 10 days before the first DMH injection and prolonged until to the 6th week after at the last injection of the carcinogen. The mean square of colon tumors was 112±18.0 mm2 and 70±11.3 mm2 in the control and PF-treated groups, correspondingly (P<0.05) (16).
Levels of advanced glycation end products (AGE) and receptors for AGE (RAGE) were examined in azoxymethane (AOM)-injected Fischer 344 rats fed a control diet (group C), a 15% linoleic acid (LA) diet (group L), a control diet with 10% glucose drink (group G), and a 15% LA diet with 10% glucose drink (groups L+G). Groups L+G showed the most pronounced increase of body weight, blood sugar and serum insulin (70). The rats in groups L+G showed the most pronounced multiplicity of aberrant crypt foci (ACF) and carcinomas with increased mucosal RAGE and AGE. IEC6 rat intestinal epithelial cells treated with AGE showed increased RAGE expression, which was inhibited by treatment with MF. In the AOM-injected rat colon cancer model (15 mg/kg s.c twice with 2-week-long interval), treatment with MF (15 mg/kg with drinking water) started after the 2nd injection of AOM, suppressed the levels of RAGE and AGE, as well as the multiplicity of ACF at the 16th week of the experiment and colon carcinomas at the 50th week in groups L+G rats (70).
The LKB1 tumor suppressor phosphorylates and activates AMPK (AMP-activated protein kinase) when cellular energy levels are low, thereby suppressing growth through multiple pathways, including inhibiting the mTORC1 kinase that is activated in the majority of human cancers. Blood glucose-lowering type 2 diabetes drugs also induce LKB1 to activate AMPK, indicating that these compounds could be used to suppress growth of tumor cells. Huang et al. (68) investigated the importance of the LKB1-AMPK pathway in regulating tumorigenesis in mice resulting from deficiency of PTEN (phosphatase and tensin homologue deleted on chromosome 10) tumor suppressor, which drives cell growth through over activation of the Akt and mTOR kinases. It was shown that inhibition of AMPK resulting from a hypomorphic mutation that decreases LKB1 expression does not lead to tumorigenesis on its own, but markedly accelerates tumor development in PTEN+/– mice. In contrast, activating the AMPK pathway by administering MF, PF or A-769662 to PTEN+/– mice significantly delayed tumor onset. LKB1 is required for activators of AMPK to inhibit mTORC1 signalling as well as cell growth in PTEN-deficient cells. Pharmacological inhibition of LKB1 and/or AMPK would be undesirable, at least for the treatment of cancers in which the mTORC1 pathway is activated. Most importantly, these results demonstrate the potential of AMPK activators—such as clinically approved MF—as anticancer agents that will suppress tumor development by triggering a physiological signalling pathway that potently inhibits cell growth.
MF (300 mg/kg of body weight per day) or PF (300 mg/kg of body weight per day) were administrated with drinking water from the age of 4 weeks. In the control LKB1fl/+PTEN+/– the first tumor was detected at the age of 4 months and 100% of mice developed tumors by 8 months. Tumors formation was markedly delayed in mice treated with MF of PF. By 6 months of age, none of the animals on PF had developed tumors. The first tumor in PF-treated group was detected at the age of 7 months and at the age of 10 months 60% of mice on PF possessed tumors. MF was less effective at in inhibiting tumorigenesis. For mice given MF, the first tumors development was detected at the age of 5 months and all mice developing tumors by 12 months (68). It worthy of note, that there were a very small number of mice in control (four males and six females), in MF- (seven males and three females) and PF-treated (five males and five females) groups. The incidence of total tumors was 100% in the control and MF groups and 80% in PF group. Intestinal polyps were observed in 2/10 control, 3/10 MF and 4/10 PF mice, whereas lymphomas were detected in 10/10 control, 10/10 MF and 7/10 PF mice.
Tomimoto et al. (67) investigated the effect of MF on the suppression of intestinal polyp formation in ApcMin/+ mice. Administration of MF (250 mg/kg with diet) did not reduce the total number of intestinal polyps, but it significantly reduced the number of polyps larger than 2 mm diameter and the average size of polyps in ApcMin/+ mice. To examine the indirect effect of MF, the index of insulin resistance and serum lipid levels in ApcMin/+ mice were assessed. These factors were not significantly attenuated by the treatment with MF, indicating that the suppression of polyp growth is not due to the indirect drug action. The levels of tumor cell proliferation as determined by 5-bromodeoxyuridine and proliferating cell nuclear antigen immunohistochemical staining and apoptosis, via transferase deoxytidyl uridine end labeling staining in the polyps of MF-treated mice, were not significantly different compared to those of control mice. Gene expression of cyclin D1 and c-myc in intestinal polyps were also not significantly different between those two groups. In contrast, MF activated AMPK in the intestinal polyps, resulting in the inhibition of the activation of mTOR, which plays an important role in the protein synthesis machinery (67).
Seven-week-old BALB/c mice were intraperitoneally injected with AOM (10 mg/kg) once a week for 2 weeks and were subdivided into three groups given diet without MF, given diet with MF (250 mg/kg/d) starting at the day of the first AOM injection or 1 week after the final AOM injection. All animals were sacrificed six weeks after the 1st AOM injection for the investigation of ACF formation) (69). After 6 weeks of AOM administration, mice fed the basal diet without MF developed 7.0±0.5 ACF per mouse, whereas treatment with MF significantly reduced this number to 2.2±0.3 and 2.9±0.4 ACF/mouse in the groups II,III, correspondingly. It was stressed that MF can effectively inhibit ACF formation after the initiation of AOM treatment. In another set of experiment, 7-week-old mice were exposed to i.p. injection of AOM once a week for 6 weeks and were given MF (250 mg/kg) with diet starting at the day of first AOM injection and finalized 32 weeks after that (69). Treatment with MF significantly inhibited colon polyp development reducing both the number polyps per mouse and the size of polyps. No significant differences in body weight or glucose concentration were observed. The BrdU and PCNA indices were decreased in mice treated with MF. Western blot analysis revealed that the phosphorylated mTOR, S6 kinase and S6 protein levels in the colonic mucosa decreased significantly in mice treated with MF. The authors believe that MF suppresses colonic epithelial proliferation via the inhibition of the mTOR pathway through the activation of AMPK. It is worthy to note that MF not affected the level of 06-methylguanine in colon or liver mice treated with AOM. These results show that MF did not affect the alkylation capacity and carcinogenicity of AOM (69).
Effect of dietary MF (100, 400, 800 to 1,600 ppm) on AOM-induced ACF development; and a dietary MF (500 and 1,000 ppm) on AOM-induced colon adenocarcinoma was studied in F344 rats (71). ACF and tumor efficacy endpoints were carried out on AOM-treated 8-week-old rats (48 per group) fed the control AIN-76A diet. Either three days (ACF Study) or 4 weeks (adenocarcinoma study) after carcinogen treatment, rats were fed the diets containing different doses of MF. ACF and colon adenocarcinomas were determined at 8 and 48 weeks after AOM-treatment, respectively. Dietary MF at 100 and 400 ppm had no significant effect on the colonic ACF formation; whereas at higher doses a significant increase was observed in the total ACF (37%) and multicrypt ACF by 43%. In long term bioassay, dietary MF at 500 and 1,000 ppm had no significant effect on adenocarcinoma incidence, multiplicity or colon tumor volume. Rats that were fed with MF fail to show significant effects on markers of cell proliferation and apoptosis in colonic tumors (71). Reasons of the discrepancies between rodent models and more so in human clinical observations on the potential beneficial effects of MF for colon cancer risk are not clear. May be quality of the drug sample used in the study was not good enough. In general, the apart this abstract all other studies reported showed the inhibitory effect of antidiabetic biguanides on MF on colon carcinogenesis.
Lung carcinogenesis
A/J mice were treated with oral MF after exposure to the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (56). MF reduced lung tumor burden by up to 53% at steady-state plasma concentrations that are achievable in humans. mTOR was only modestly inhibited in lung tumors. To test whether intraperitoneal administration of MF might improve mTOR inhibition, authors injected mice and assessed biomarkers in liver and lung tissues. Plasma levels of MF were significantly higher than oral administration after injection. In liver tissue, MF activated AMPK and inhibited mTOR. In lung tissue, MF did not activate AMPK but inhibited phosphorylation of IGF-I receptor/insulin receptor (IGF-1R/IR), Akt, ERK and mTOR. This suggested that MF indirectly inhibited mTOR in lung tissue by decreasing activation of IGFIR/IR and Akt upstream of mTOR. Based on these data, the authors repeated the NNK-induced lung tumorigenesis study using intraperitoneal administration of MF. MF decreased tumor burden by 72%, which correlated with decreased cellular proliferation and marked inhibition of mTOR in tumors. These studies show that MF prevents tobacco carcinogen-induced lung tumorigenesis (56). Quinn et al. (57) used liver IGF-1-deficient (LID) mice for study the effect of MF on lung carcinogenesis. These mice have a 75% reduction in circulating IGF-1 levels and were backcrossed in the A/J background seven generation to make them susceptible to NNK. Eight-week-old mice were given 3 weekly injections of 100 mg/kg NNK. One week after the last injection of the carcinogen mice were randomized into a control group and a group that received 5 mg/mL MF dissolved in drinking water. Lung tumors developed in 67% wild type mice and in 40% of LID mice. Treatment with MF decreased by 64% lung tumor multiplicity and by 77% tumor burden in wild type mice. LID mice were more susceptible to inhibitory effect of MF. The drug reduced tumor multiplicity by 75% and burden—by 85% as compared with control group in LID mice.
Sixty-one male 129/Sv mice were exposed to a single intraperitoneal injection of urethane dissolved in 0.9% neutral saline at the dose of 1 g/kg of the body weight (58). Starting the day after the carcinogen injection, mice of one group were given 5 days a week of MF with drinking water (600 mg/L) at a dose of 200 mg/kg. Control mice were given drinking water without MF. Six months after the urethane treatment, the experiment was finalized and all mice were sacrificed. Tumors (lung adenomas and thymic lymphomas) developed in 29 of 30 control mice (96.7%) and in 25 of 31 mice exposed to MF (80.7%; P<0.05). Solid or trabecular lung adenomas developed in 90% of the control mice and in 77% of the mice in the group given MF (P<0.119) and has no effect on multiplicity of lung tumors. Thymic lymphomas were detected in 10% of control mice and in 3% of MF-treated animals (58). These data show the slight inhibitory effect of MF on urethane-induced tumorigenesis in mice.
Neurogenic and kidney transplacental carcinogenesis
A decrease of glucose utilization in the oral glucose tolerance test was found in the 3-month-old female progeny of rats exposed to NMU on the 21st day of pregnancy (11,13). The serum insulin level was not different from the control, but the cholesterol level was higher in offspring of NMU-treated rats as compared with the control. Postnatal treatment with BF (5 mg/rat, per os, 5 times a week) until the natural death of animals, started from age 2 months significantly inhibited the development of malignant neurogenic tumors in rats transplacentally exposed to NMU. Total number of rats with any malignancies was 54.2% in control group and 27%—in rats treated with BF, whereas number of rats with tumours of nervous system was 33.4% and 9.5%, respectively. Similar results have been observed in rats exposed transplacentally to N-nitrosoethylurea (NEU) and postnatally to PF (18). The authors observed decreased development of nervous system and renal tumors induced transplacentally with NEU.
Radiation carcinogenesis
The treatment with PF also inhibited the carcinogenesis induced by a single total-body X-ray irradiation in rats (17). Female rats at the age of 3 months were exposed to a single dose 4 Gy of total-body X-ray irradiation, and starting next day after irradiation were given per os tap water (control) or PF 2 mg/rat 5 times a week until natural death. In 6 months after irradiation, irradiated rats revealed hyperglycaemia, hyperinsulinemia and hypertriglyceridemia in parameters similar to 24-month-old non-irradiated rats. Total tumor incidence in X-ray irradiated alone rats was 78%, whereas in the group treated with PF—48%. PF inhibited the development of pituitary, thyroid, mammary tumors, decreased their multiplicity as compared with non-irradiated controls.
Thus, antidiabetic drugs inhibit spontaneous and induced carcinogenesis in rodents. The effect did not depend on type of a carcinogen, tumor localization and histogenesis: it was observed in the relation of epithelial, mesenchymal, neurogenic and lymphoid malignancies developed spontaneously or induced by various chemical carcinogens (polycyclic aromatic hydrocarbons, nitroso compounds), irradiation, virus, transgene.
Effect of dose and route of administration of MF
It was shown that after a single dose a peak plasma concentration of MF reported in diabetic patients range from 0.5 to 2 µg/mL (4 to 15 µM) (74). After repeated administration, MF does not accumulate in the plasma and did not bind to plasma proteins. Pollak (3) stressed that most of the laboratory evidence for antineoplastic activity of MF was observed at higher exposure levels than achieved during diabetes treatment in patients.
We believe that it absolutely correct in relation to the majority of in vitro studies (sometime the dose of MF was as high as 50 mM), but only partly correct in relation to in vivo studies. Quinn et al. (57) studied biodistribution of 14C-labeled MF in mice in 1 hour after intraperitoneal injection (250 mg/kg) or gavage (5 mg/mL), and in mice allowed to drink oral MF dissolved in water (5 mg/mL) ad libitum during 5 days. It was observed practically similar patterns of MF distribution after a single intraperitoneal or gavage administration with MF levels about 4.5 times higher in liver than in lungs. In plasma of mice that were allowed to drink MF ad libitum, absolute levels of MF was 6.1 µM whereas in mice exposed to a single i.p. injection or gavage—29.1 and 12.27 µM respectively. It’s easy to show that doses of MF less than 500 mg/kg in mice yielded plasma level of MF practically similar to that in diabetic patients. Our calculation has shown that used in our experiments dose of MF 100 mg/kg equal to 300 mg/m2 of the surface area. Recalculation for humans gives in average 510 mg/m2, that is much less that commonly used in clinical practice (1.0-2.5 g per day) (29,39). We believe that studies on the effect of various routes of MF administration at various doses levels will very helpful for a search most optimal regimens for prevention and treatment of cancer.
Mechanisms of effects of antidiabetic biguanides on aging and cancer
It is noteworthy that studies of MF distribution in mice showed a measurable accumulation of 14C MF in brain tissue 2 hours after a single oral dose (150 mg/kg) (75). The studies revealed that orally administered MF increases brain AMPK activation. This suggests that MF crosses the blood-brain barrier and exerts a pharmacological effect in the intact brain. Data on molecular mechanisms of the inhibitory effect of biguanides on tumor growth have been discussed in several comprehensive reviews (1-4,32-34,41,42,76-80). The analysis of this fast growing research direction is behind the aim of our paper. It’s worthy to note that biguanides may affect specific metabolic processes in transformed cells directly and indirectly involving alteration in host environment (32-34,41).
Antidiabetic drugs and cancer risk in diabetic patients
There is evidence that insulin resistance or some other aspect of type 2 diabetes may promote breast and some other cancers (1-4). Biguanides were used as a component of the so-called metabolic rehabilitation of breast and colon cancer patients (1,25-28). MF is the most prescribed drug for treatment of type 2 diabetes mellitus patients. There are almost hundred ongoing or upcoming clinical studies on effect of MF in cancer patients listed in www.ClinicalTrials.gov and in the International Clinical Trials Registry Platform (2). It should be stressed that some of them focused on cancer preventive effect of the drug. It gives a hope that the scientific community will finally able with light heart to recommend MF for cancer prevention not only in type 2 diabetics but also in peoples without clinically manifested diabetes, in groups of risk and in “normal” persons. It is worthy to note that Nobel Prize J. Watson stressed that highly focused new drug development should be initiated towards finding compounds beyond MF that selectively kill stem cells (81,82).
Conclusions
MF use in diabetic patients has been associated with reduced cancer glucose metabolism and insulin signaling pathway, as well as such important longevity-related parameters as fertility and resistance to oxidative stress and tumorigenesis. Furthermore, there is evidence that MF may have insulin-independent direct effects on cancer cells, acting as a mTOR inhibitor (37-42). It was suggested that this dual action of MF (insulin reduction and mTOR inhibition) makes it a particularly attractive target for evaluation in breast cancer (76,77,83) as well as some other cancers, e.g., colon cancer. It seems that there are a lot of clinical trials suggest causal relationships established between us of antidiabetic biguanides and the prevention of human cancer. At the same time, there are many experimental evidences of causal relationships between antidiabetic biguanides and a decreased incidence and/or multiplicity of neoplasms in rodents. Thus, available data on effects of antidiabetic biguanides on cancer both as anti-tumor growth and as cancer preventive agent allow recommending to evaluate its efficacy according to experience and rules of the International Agency for Research on Cancer Monograph programme adopted for IARC Handbooks of Cancer Prevention series (http://www.iarc.fr/en/publications/list/handbooks/index.php).
Acknowledgements
This paper was supported in part by a grant 6538.212.4 from the President of the Russian Federation. The author is very thankful to I.G. Popovich and M.A. Zabezhinski for critical reading of the manuscript and valuable comments.
Disclosure: The author declares no conflict of interest.
References
- Berstein LM. eds. Biguanides: An Expansion to Practical Oncology (Past and Present). St.Petersburg: Aesculap, 2010.
- Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469-80. [PubMed]
- Pollak M. Potential applications for biguanides in oncology. J Clin Invest 2013;123:3693-700. [PubMed]
- Schäfer G. Biguanides. A review of history, pharmacodynamics and therapy. Diabete Metab 1983;9:148-63. [PubMed]
- Dilman VM. Age-associated elevation of hypothalamic threshold to feedback control and its role in development, aging and disease. Lancet 1971;1:1211-9. [PubMed]
- Dil’man VM, Bershteĭn LM, Zabezhinskiĭ MA, et al. Effect of phenformin on mammary gland tumor induction in rats. Vopr Onkol 1974;20:94-8. [PubMed]
- Dil’man VM, Sofronov BN, Anisimov VN, et al. Phenformin elimination of the immunodepression caused by 1,2-dimethylhydrazine in rats. Vopr Onkol 1977;23:50-4. [PubMed]
- Dilman VM, Berstein LM, Zabezhinski MA, et al. Inhibition of DMBA-induced carcinogenesis by phenformin in the mammary gland of rats. Arch Geschwulstforsch 1978;48:1-8. [PubMed]
- Dil’man VM, Anisimov VN. Increase in longevity and a decrease in the frequency of tumors in C3H/Sn mice under the influence of fenformin and diphenin. Dokl Akad Nauk SSSR 1979;245:753-7. [PubMed]
- Dilman VM, Anisimov VN. Effect of treatment with phenfromin, dyphenylhydantoin or L-DOPA on life span and tumor incidence in C3H/Sn mice. Gerontology 1980;26:241-6. [PubMed]
- Alexandrov VA, Anisimov VN, Belous NM, et al. The inhibition of the transplacental blastomogenic effect of nitrosomethylurea by postnatal administration of buformin to rats. Carcinogenesis 1980;1:975-8. [PubMed]
- Anisimov VN. Effect of buformin and diphenylhydantoin on life span, estrus function and spontaneous tumor incidence in female rats. Vopr Onkol 1980;26:42-48. [PubMed]
- Anisimov VN, Aleksandrov VA, Belous NM, et al. Inhibition of the transplacental blastomogenic effect of N-nitrosomethylurea in rats by buformin. Biull Eksp Biol Med 1980;89:88-90. [PubMed]
- Anisimov VN, Belous NM, Vasilyeva IA, et al. Inhibitory effect of phenformin on the development of mammary tumors induced by N-nitrosomethylurea in rats. Exp Onkol 1980;2:40-3.
- Anisimov VN, Ostroumova MN, Dil’man VM. Inhibition of the blastomogenic effect of 7,12-dimethylbenz(a)anthracene in female rats by buformin, diphenin, a polypeptide pineal extract and L-DOPA. Biull Eksp Biol Med 1980;89:723-5. [PubMed]
- Anisimov VN, Pozharisski KM, Dilman VM. Effect of phenformin on the blastomogenic action of 1,2-dimethylhydrazine in rats. Vopr Onkol 1980;26:54-8. [PubMed]
- Anisimov VN, Belous NM, Prokudina EA. Inhibition by phenformin of the radiation carcinogenesis in female rats. Exp Onkol 1982;4:26-9.
- Bespalov VG, Aleksandrov VA. Influence of anticarcinogenic agents on the transplacental carcinogenic effect of N-nitroso-N-ethylurea. Biull Eksp Biol Med 1985;100:73-6. [PubMed]
- Cohen MH, Strauss BL. Enhancement of the antitumor effect of 1,3-bis(2-chloroethyl)-l-nitrosourea (BCNU) by phenylethylbiguanide (phenformin). Oncology 1976;33:257-9. [PubMed]
- Dilman VM, Anisimov VN. Potentiation of anti-tumor effect of cyclophosphamide and hydrazine sulfate by treatment with the antidiabetic agent, 1-phenylethylbuguanide (phenformin). Cancer Lett 1979;7:357-61. [PubMed]
- Dil’man VM, Anisimov VN. An increase in hypothalamic sensitivity to the inhibiting effects of estrogens caused by use of L-DOPA, diphenine, epithalamin and phenformin in old rats. Biull Eksp Biol Med 1975;80:96-8. [PubMed]
- Dilman VM, Anisimov VN. Hypothalamic mechanisms of ageing and of specific age pathology – I. Sensitivity threshold of hypothalamo-pituitary complex to homeostatic stimuli in the reproductive system. Exp Gerontol 1979;14:161-74. [PubMed]
- Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev 2007;CD005552. [PubMed]
- Nestler JE, Stovall D, Akhther N, et al. Strategies for the use of insulin-sensitizing drugs to treat infertility in women with polycystic ovary syndrome. Fertil Steril 2002;77:209-15. [PubMed]
- Dilman VM, Berstein LM, Ostroumova MN, et al. Metabolic immunodepression and metabolic immunotherapy, an attempt of improvement in immunologic response in breast cancer patients by correction of metabolic disturbances. Oncology 1982;39:13-9. [PubMed]
- Dilman VM, Berstein LM, Yevtushenko TP, et al. Preliminary evidence on metabolic rehabilitation in cancer patients. Arch Geschwulstforsch 1988;58:175-83. [PubMed]
- Berstein LM, Evtushenko TP, Tsyrlina EV. Comparative study of 5- and 10-year-long results of the metabolic rehabilitation of cancer patients. In: Hanson KP, Dilman VM. eds. Neuroendocrine System, Metabolism, Immunity and Cancer (Clinical Aspects). St.Petersburg: N.N. Petrov Research Institute of Oncology Publ, 1992;102-12.
- Berstein LM. Modern approach to metabolic rehabilitation of cancer patients: biguanides (phenformin and metformin) and beyond. Future Oncol 2010;6:1313-23. [PubMed]
- Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol 2005;40:685-93. [PubMed]
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [PubMed]
- Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylurea or insulin. Diabetes Care 2006;29:254-8. [PubMed]
- Del Barco S, Vazquez-Martin A, Cufí S, et al. Metformin: multi-faceted protection against cancer. Oncotarget 2011;2:896-917. [PubMed]
- Pollak M. Insulin-like growth factor-related signaling and cancer development. Recent Results Cancer Res 2007;174:49-53. [PubMed]
- Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer. Doses, mechanisms and the dandelion and hermetic phenomena. Cell Cycle 2010;9:1057-64. [PubMed]
- Taubes G. Cancer prevention with a diabetes pill? Science 2012;335:29. [PubMed]
- Anisimov VN, Semenchenko AV, Yashin AI. Insulin and longevity: antidiabetic biguanides as geroprotectors. Biogerontology 2003;4:297-307. [PubMed]
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760-74. [PubMed]
- Anisimov VN. Metformin and rapamycin are master-keys for understanding the relationship between cell senescent, aging and cancer. Aging (Albany NY) 2013;5:337-8. [PubMed]
- Anisimov VN. Metformin: Do we finally have an anti-aging drug? Cell Cycle 2013;12:3483-9. [PubMed]
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol 2013;87:201-23. [PubMed]
- Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discovery Today 2007;12:218-24. [PubMed]
- Blagosklonny MV. Common drugs and treatments for cancer and age-related diseases: revitalizing answers to NCI’s provocative questions. Oncotarget 2012;3:1711-24. [PubMed]
- Anisimov VN, Egormin PA, Piskunova TS, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 2010;9:188-97. [PubMed]
- Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 2008;7:2769-73. [PubMed]
- Bojkova B, Orendas P, Garajlova M, et al. Metformin in chemically-induced mammary carcinogenesis in rats. Neoplasma 2009;56:269-274. [PubMed]
- Zhu Z, Jiang W, McGinley JN, et al. Comparison of metformin, pheformin and buformin in mammary carcinogenesis in non-obese, non-diabetic rats. Proc of the 104th Annual Meeting of the AACR, 2013, Washington DC, Abstract 2272.
- Deriabina ON, Plotnikova NA, Anisimov VN. Melatonin and metformin inhibits skin carcinogenesis induced by benzo(a)pyrene in mice. Vopr Onkol 2010;56:583-7. [PubMed]
- Man’cheva TA, Demidov DV, Plotnikova NA, et al. Melatonin and metformin inhibit skin carcinogenesis and lipid peroxidation induced by benz(a)pyrene in female mice. Bull Exp Biol Med 2011;151:363-5. [PubMed]
- Checkley LA, Rho O, Angel JN, et al. Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prev Res (Phila) 2014;7:54-64. [PubMed]
- Vinnitskii VB, Iakimenko VA. Effect of fenformin, L-DOPA and para-chlorophenylalanine on the immunological reactivity and chemical carcinogenesis in BALB/c mice. Vopr Onkol 1981;27:45-50. [PubMed]
- Deriabina ON. eds. Inhibitory effect of melatonin and metformin on carcinogenesis induced by benzo(a)pyrene in various tissues of female mice. St.Petersburg: Ph Diss, NN Petrov Res Inst Oncology, 2010.
- Bioassay of Phenformin for Possible Carcinogenicity. Natl Cancer Institute. Carcinogenesis. Technical Report Series No. 7. US DHEW. Public Health Service, NIH, 1977.
- Erdemoglu E, Güney M, Giray SG, et al. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol 2009;145:195-9. [PubMed]
- Anisimov VN, Piskunova TS, Popovich IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2:945-58. [PubMed]
- Deriabina ON, Plotnikova NA, Kharitonova TV, et al. Melatonin and metformin inhibits development of cervico-vaginal tumors induced by benzo(a)pyrene in mice. Morphol. Vedomosti. 2010;2:36-41.
- Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066-76. [PubMed]
- Quinn BJ, Dallos M, Kitagawa H, et al. Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila) 2013;6:801-10. [PubMed]
- Popovich IG, Piskunoiva TS, Tyndyk ML, et al. Effect of metformin on urethane-induced lung cacinognesis in mice. Vopr Onkol 2012;58:549-53. [PubMed]
- Vitale-Cross L, Molinolo AA, Martin D, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5:562-73. [PubMed]
- Schneider MB, Matsuzaki H, Harorah J, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001;120:1263-70. [PubMed]
- Mohammed A, Janakiram NB, Brewer M, et al. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol 2013;6:649-59. [PubMed]
- Hou M, Venier N, Sugar L, et al. Protective effect of metformin in CD1 mice placed on a high carbohydrate-high fat diet. Biochem. Biophys Res Commun 2010;397:537-42. [PubMed]
- Bhalla K, Hwang BJ, Dewi RE, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544-52. [PubMed]
- Afzal M, Kazmi I, Gupta G, et al. Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm J 2012;20:365-70. [PubMed]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013;4:2192. [PubMed]
- Tajima K, Nakamura A, Shirakawa J, et al. Metformin prevents liver tumorigenesis induced by high-fat diet in C57Bl/6 mice. Am J Physiol Endocrinol Metab 2013;305:E987-98. [PubMed]
- Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci 2008;99:2136-41. [PubMed]
- Huang X, Wullschleger S, Shpiro N, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J 2008;412:211-21. [PubMed]
- Hosono K, Endo H, Takahashi H, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by AMP-activating protein kinase. Mol Carcinogenesis 2010;49:662-71.
- Shimomoto T, Luo Y, Ohmori H, et al. Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J Gastroenterol 2012;47:1073-83. [PubMed]
- Rao CV, Janakiram NB, Mohammed A, et al. Lack of chemopreventive effects of metformin in azoxymethane-induced rat colon carcinogenesis. Cancer Prev Res 2011;4:B42.
- Anisimov VN, Berstein LM, Popovich IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148-57. [PubMed]
- Zhang Q, Celestino J, Schmandt R, et al. Chemopreventive effects of metformin on obesity-associated endometrial proliferation. Am J Obstet Gynecol 2013;209:24.e1-24.e12.
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 2011;50:81-98. [PubMed]
- Beckman R. Biguanide (Experimental Teil). In: Maske H. eds. Handbook of Experimental Pharmacology, Vol 29. Berlin: Springer-Verlag, 1971;439-596.
- Pollak M. Insulin-like growth factor-related signaling and cancer development. Recent Results Cancer Res 2007;174:49-53. [PubMed]
- Vazquez-Martin A, Oliveras-Ferraros C, Cufi S, et al. Metformin and energy metabolism in breast cancer: from insulin physiology to tumour-initiating stem cells. Curr Mol Med 2010;10:674-91. [PubMed]
- Wysocki PJ, Wierusz-Wysocka B. Obesity, hyperinsulinemia and breast cancer: novel targets and a novel role for metformin. Expert Rev Mol Diagn 2010;10:509-19. [PubMed]
- Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253-70. [PubMed]
- Parkhitko AA, Favorova OO, Khabibullin DI, et al. Kinase mTOR: Regulation and Role in Maintenance of Cellular Homeostasis, Tumor Development, and Aging. Biochemistry (Mosc) 2014;79:88-101. [PubMed]
- Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol 2013;3:120144. [PubMed]
- Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA 2013;110:972-7. [PubMed]
- Goodwin PJ. Insulin in the adjuvant breast cancer setting: A novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol 2008;26:833-4. [PubMed]


