
Stem cells and stem cell-derived extracellular vesicles in acute and chronic kidney diseases: mechanisms of repair
Introduction
The kidney has a very complex structure represented by several, highly specialized cells composed in an interwoven architecture. The different microscopic structures composing the nephron include the glomerulus acting as filtrating unit and the tubules involved in active release and absorption of molecules. The kidney far from being a simply “filtering device” is capable of sensing variations in plasma osmosis and volume by correctly interpreting sensor-guided signals. Glomeruli and tubules play in a harmonized vascularized environment embedded in the interstitium. The kidney is composed by a large vascular bed represented by a unique fenestrated endothelial lining in the glomerulus and an extensive peritubular capillary network. Epithelial cells differentiated into several types with specialized functions line the walls of glomerular capillaries (podocytes) and the wall of membranes of the proximal and distal tubules. Finally, the kidney is a relevant endocrine organ in the regulation of fluid volume, blood pressure, calcium-phosphate metabolism, and erythropoiesis. It is evident that for its central role in the maintenance of the body’s interne milieu, the failing kidney induces important repercussions for many other vital organs of the body. Likewise, its central role in receiving at least 1/4th of the cardiac output is subjected to many types of insults that may markedly reduce or even stop the function of the kidneys.
Classically, acute and chronic renal failure have long been described and now renamed as acute kidney injury (AKI) and chronic kidney disease (CKD). New concepts are emerging in the pathophysiology of kidney diseases. AKI is often caused by triggering factors (e.g., toxic, ischemic, immunologic) either individually or combined such as in sepsis (inflammation and hypoxia) and it is initiated at a defined time. Several experimental models of AKI have provided deep insight and have convincingly shown important proof-of-concepts of therapeutic relevance over the years. CKD is now considered a slowly developing disease with often an insidious course, lasting many years whereby co-morbidities (e.g., diabetes, hypertension, dysmetabolic syndrome) may act as worsening factors. However, although AKI and CKD initially have been described as different entities, it has become increasingly evident that even a single event of AKI may lead to a higher predisposition to develop a progressive CKD.
AKI: epidemiology and economic burden
AKI is still one of the most puzzling renal syndromes, with incidence rates fluctuating from 0.9% to 20% and the whole mortality between 25% and 80% (1). These divergences are most probably due to large differences in AKI designation, case-mix, and experience with AKI therapy and its associate pathology [as reviewed by the Acute Dialysis Initiative in (2)]. Nonetheless, we still face a large uncertainty in the early diagnosis of AKI due to the lack of specificity of diagnostic markers and late intervention [reviewed in (3)]. The need of biomarkers has led to the search for novel bio-molecules for early detection of AKI. AKI is also frequently associated with the heart, liver, lung and cardiovascular disease based on organ cross-talk (4). Selected urine biomarkers of kidney injury were independently related with higher incidence of heart failure, cardiovascular disease events, and death in the CRIC study (5). Among the biomarkers examined, only KIM-1/Cr was associated with each outcome (5).
An important percentage of patients with AKI needs dialysis (6). The incidence of mortality in patients who necessitate dialysis for AKI in the intensive care units (ICU) is of 50–60%, approximately twofold that of equivalent patients without AKI (7,8). Care is increased several-fold, resource intense, and technically involved. Patients with AKI necessitating dialysis therapy need to be treated competently and effectively. A recent cost analysis of the different renal replacement therapies has been published (9).
Pathophysiology of AKI
The glomerular cells are composed of mesangial, endothelial and epithelial cells (podocytes). Podocytes allow the glomerular filtration barrier to be stably maintained thanks to their highly differentiated post mitotic phenotype. Originally, cells in the adult kidney were considered to be unable to proliferate after full completion of the development. However, recently kidney cells have been shown to be able to regenerate and to repair themselves throughout life (10). Podocytes may undergo various kinds of stress, mechanical, oxidative or immunologic. Podocytes have a relevant ability to strain stress. For a long time, our knowledge on podocyte-associated injury has been only related to the presence of foot effacement by electron microscopy. However, it has nowadays become increasingly clear that the application of gene profiling and single cell analysis (11) will help in the recognition of certain gene dis-regulation and molecule expression. The latter may be not only paradigmatic signs of the disease but also potential targets of therapeutic intervention (12). Glomerular podocytes, endothelial and mesangial cells and tubular epithelial cells are particularly vulnerable when exposed to antibodies, cytokines such as in sepsis, to exogenous radiocontrast agents and aminoglycosides or to endogenous toxins such as myoglobin and activated complement, or to ischemia (13). AKI recovery depends on the capacity of renal tubules to regenerate and recuperate normal function and on the entity of the injury. In parallel, patient age may lead to requirement for long-term dialysis and to increased mortality (14). Widespread necrosis and exfoliation of tubular epithelial cells are commonly seen in AKI (15). Replacement of necrotic tubular cells with functional tubular epithelium is observed during recovery (16). Failure of replacing injured epithelial and endothelial cells may lead to tubule-interstitial fibrosis and CKD (16). After injury, tubular cells, may acquire a mesenchymal phenotype after de-differentiation thus becoming capable of replacing necrotic or apoptotic cells and of repopulating areas of denuded tubular basement membrane. Subsequent differentiation into mature epithelial cells will lead to recovering tissue integrity. The process of repair is based on local paracrine mechanisms including the release of growth factors such as insulin-like growth factor-1, epidermal growth factor and hepatocyte growth factor (17). Furthermore, several G protein-coupled receptors are critical in both renal physiology and pathophysiology. Upregulation of Gpr97 occurs in experimental AKI and in AKI patients. Deficiency of Gpr97 was shown to reduce the expression of semaphorin 3A, which in AKI is considered a biomarker of renal injury (18).
Finally, a relevant role in coordination of renal repair has been also ascribed to bone marrow-derived and resident stem/progenitor cells (19).
Pathophysiology of CKD
CKD has a prevalence of approximately 15% in the general population and it is frequently associated with cardiovascular disease and/or diabetes (20). Currently, the expenditure has raised up to $35 billion annually for CKD care (21). The better understanding of the risk factors associated to progression of CKD from type 2 to type 4 is an area of recent, great interest especially in the light of the fact that these patients are still too often lost to follow-up by nephrologists. More advances in this area of pathophysiology, novel biomarkers and overall a higher clinical readiness are expected to reduce consistently the incidence of CKD. Today, the development of CKD is mainly associated to comorbidities such as hypertension, cardiovascular disease, diabetes mellitus, pre-existing renal dysfunction or glomerular and/or tubular diseases. These co-morbid factors, alone or in association with AKI or even AKI alone may lead to structural damage depending on a series of concurrent, multiple pathophysiological alterations including glomerular hyper-filtration, proteinuria, glomerular- and tubular-sclerosis, interstitial inflammation leading to progressive decline of the renal function. Moreover, it has been shown that tubular/interstitial complement activation has a critical role in CKD progression (22). The tubular/interstitial injury is considered an independent factor of CKD progression also for glomerular diseases (23,24).
The pattern of morbidity and mortality is changing worldwide. Before and through the 20th century, infectious diseases or heavy exposure to toxic agents were among the first causes of both morbidity and mortality and still are in the undeveloped countries. The era of non-communicable, noninfectious affections is now appearing as the new challenge. Diabetes is the single principal cause of CKD (25). Treatments that may delay progression of CKD in diabetes will greatly impact on clinical outcomes and will reduce the cost of CKD care. Even if the diet may partially slow down CKD, until now only therapy with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may moderately decrease CKD progression in diabetes (26). An improved glycemic control by the sodium-glucose transport protein-2 inhibitors (27) may give even more hope that in the future the decline in renal function may be kept under a tighter control leaving more space for innovative cell therapies.
Experimental models of acute and chronic kidney injury
Adequate animal models are needed to discover new biomarkers for disease staging and therapy individualization as well as dosing and route of administration. Experimental models of renal injury hardly resemble the human setting as many factors may interfere with the renal response including toxins, intrinsic vaso-constrictive renal response, aging etc., that are difficult to reproduce in a single experimental model. In this review, we will refer only to the models that have been used to prove a potential role of stem cells and stem cell-derived extracellular vesicles (EVs) (Table 1). To the interested reader, excellent reviews offer a clear picture on the experimental models of renal disease (50,51).
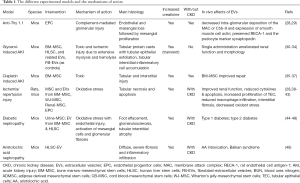
Full table
In Table 1 we summarized the different experimental models, their categorization (whether acute or chronic or both), and their mechanisms of action. The use of immunodeficient mice as well as strain-dependent variation in sensitivity need to receive full awareness.
Stem cells: novel insights in the mechanisms of renal repair
Important progress has been made in recent years on the identification of embryonic precursor cells and adult progenitor cells of the kidney. Due to ethical concerns related to the utilization of embryonic cells and the difficulty to access embryonic tissues, researchers focused on adult stem cells such as bone marrow- and adipose-tissue derived mesenchymal stem cells, endothelial progenitor cells and human liver-derived stem cells.
Self-renewing and differentiation into multiple cell types are features of all stem cells. Stem cells present in the bone marrow are capable to generate the cellular components of various tissues (52-54). In fact, while blood cells originate from the hematopoietic stem cell compartment, osteoblasts, chondrocytes, adipocytes and myocytes originate from mesenchymal stem cells (MSC) (55). Resident stem cells have been found in different tissues such as the central nervous system (56), the retina (57), the skeletal muscle (58), the liver (59) and the kidney (60). Tissue stem cells may differentiate into cells of the same tissue, but also into cells of diverse embryonic lineages (55). Resident stem cells cooperate to the growth of organs after birth and to the tissue cell turnover mostly in skin, intestine and kidney, all these organs sharing a high level of cell turnover. Adult stem cells could be critical also for tissue repair following damage (61).
Even if new nephron genesis does not occur after birth, it has been demonstrated that during life, kidneys may renew the cellular compartments and, to a certain extent, undergo repair (10). In the presence of an insult, however, the regenerative potential may be overcome. Adult stem cells are regarded as a promising alternative in the field of kidney regeneration following AKI.
Many studies in the literature have shown that MSC exert beneficial effects at least partly through a paracrine action as opposed to engraftment within damage tissue (62). An important prerequisite for the homing of stem cells to a specific site is the presence of injury in order to exert their regenerative effects (30).
Several experimental studies described renal improvement by MSC administration both in AKI and CKD conditions (31,35,38,63). Systemically injected MSC were able to reach the damaged tissues and act within the microenvironment. The homing of MSC into the site of injury was triggered by stromal derived factor with the activation of the CXCR4 chemokine receptor (64,65). Additionally, the interaction between CD44 and glycosaminoglycan hyaluronan correlated with the ability of MSC to migrate in the extracellular matrix (30) as demonstrated by a decrease in homing of MSC within the damaged kidney when the loss of CD44 function was induced by mutant MSC (30). Only a small number of systemically injected MSC engrafted within the injured tubules (30,31,35). In a rat experimental model of IRI, Toegel et al. (39) showed a differentiation independent mechanism of protection by MSC. In fact, MSC first concentrated in the peritubular capillaries, localized in the interstitium 24h after injection. No MSC trans-differentiation into tubular epithelial cells was present 3 days later. In another study performed in the glycerol-induced AKI model, Hauser et al. (32) demonstrated the vanishing of MSC from the renal tissues 5 days post injection pointing toward a paracrine action exerted by MSC in the improvement of the renal function. In effect, Bi et al. (66) could prove that in a murine AKI model induced by cisplatin, the intraperitoneal injection of MSC conditioned medium was capable to reproducing the positive effects exerted on the kidney by the cells with enhanced tubular cell survival and reduced apoptosis. Notably, it has been shown that insulin-like growth factor-1 and vascular endothelial growth factor were considered mediators of kidney repair (67,68). Indeed, it has been demonstrated that insulin-like growth factor-1 gene silencing in MSC in the cisplatin AKI model and the vascular endothelial growth factor knockdown by small interfering RNA in IRI model decreased the positive effects of MSC on renal repair (67,68). Moreover, MSC allowed to ameliorate kidney function in a model of renal failure induced in Sprague-Dawley rats by 5/6 nephrectomy (63).
Of interest, Baban et al. (69) showed the influence of the diabetic microenvironment on circulating and kidney stem cells leaving space to the concern on how it could also influence paracrine mechanisms of repair. Collectively, their results propose that the diabetic environment negatively influences the survival of stem cell subsets ensuing in increased apoptosis. For stem cell-based therapies, it remains to be established how the environment may hamper allogeneic stem cells and their paracrine function.
The favorable properties of MSC in preclinical studies performed in animal models both in acute and chronic renal injuries encouraged the performance of phase I and II clinical trials to study safety and efficacy of MSC therapy for kidney disease (70). One phase I trial in 2013 (NCT00733876) evaluating safety and efficacy of bone marrow-derived MSC injection in 16 patients submitted to on-pump cardiac surgery characterized by high risk of AKI, showed that therapy with MSC was safe and protective for the kidney (71). However, these results were not confirmed by a subsequent phase II study (NTC01602328) performed on 156 patients who developed AKI 48 hours after surgery. The intra-aortic administration of allogeneic MSC was shown to be safe and well tolerated but did not improve kidney function and mortality (72). Other clinical studies evaluated safety and efficacy of therapies with MSC for CKD. The NCT02166489 phase I trial in 2014 aimed at testing the safety and tolerability of autologous bone marrow-derived MSC treatment in 6 CKD patients, revealed the absence of adverse effects over 1-year follow-up but without exerting beneficial effects on kidney function (73). Characterization of clinical grade homogeneous populations of adipose tissue-derived MSC has been done (74). Adipose tissue derived MSC have been used in a phase I trial in 2013 (NCT01840540) with the purpose to assess the safety and toxicity of these cells in CKD patients suggesting the possibility for using this source of MSC for clinical therapy. For other clinical trials including CKD patients using autologous bone marrow-derived MSC (NCT02195323) and adipose tissue derived MSC (NCT02266394) the results are not yet published (75). Finally, a phase I/II clinical trial designed to develop new treatments for diabetic nephropathy using MSC therapies (NCT02585622) is still ongoing. This trial is evaluating safety and efficacy of therapy with bone marrow-derived MSC in diabetic nephropathy.
EVs: an alternative to stem cells
EVs collectively including exosomes, ectosomes and microvesicles, are produced by all living cells and have attracted the interest of many investigators for their role in affecting recipient cells through their complex cargoes of biological molecules such as lipids, proteins and nucleic acids. Many studies show that transitory cell localization in the damaged tissues may be sufficient to support functional and regenerative actions suggesting the involvement of paracrine mediators. Beside secretion of growth factors, also EVs actively released from cells may represent a mechanism of cross talk between cells (76-78).
The vesicles originated from the endosomal membrane compartment after fusion with the cell membrane are named exosomes, whereas EVs released from the surface of activated cells are named ectosomes or microvesicles (79-81). EVs may influence target cells directly or by conveying their cargo (77-79).
Embryonic stem cells are an abundant source of EVs that may sustain stem cell self-renewal and expansion (82). In addition, Ratajczak et al. (83) showed that a horizontal transfer of mRNA and proteins mediated by embryonic stem cell EVs can reprogram the haematopoietic progenitors. Deregibus et al. (84) showed that the horizontal transfer of mRNA mediated by EVs released from endothelial progenitor cells is associated with the activation of the regenerative programs. Valadi et al. (85) have shown that beside mRNA, functional miRNA can be transferred through EVs and represent a more general mechanism of interchange of genetic material among cells.
The content of the EV cargo is also dependent on the state of the releasing cell (whether in physiologic or stressed conditions). In vitro the production of EVs from adipose tissue-derived MSC may lead to different composition of miRNAs and soluble factors that may induce opposite biological effects (pro- vs. anti-angiogenetic) (86). EVs carry the hallmarks of the secreting cell type. In the kidney, EVs have a role in renal physiology including development, control of ion transport, removal of bio-waste, proximal-to-distal communication, regulation of inflammation and immune reaction in renal diseases (87,88). Different renal cells release EVs, which may be recovered in the urines leading to new perspectives in the field of biomarkers for diagnosis and severity assessment in renal diseases (89). Due to their potential as mediators of cellular signaling, their regenerative activity has emerged. The commonest experimental models used in pre-clinical studies reproduce glomerular immunologic damage, mixed toxic-ischemia, and ischemia reperfusion injury (IRI). Recently, EVs derived from different adult stem and progenitor cells were able to support tissue regeneration in a variety of experimental models by favoring a transient dedifferentiation and proliferation of tissue resident cells survived to tissue injury (Table 1). For instance, it has been shown that EVs induce AKI recovery by reducing tubular apoptosis and promoting tubular cell proliferation (90). IRI is a frequent pathophysiological condition of AKI. In the clinical setting, hypotension, acute hypovolemia, hypoxia frequently seen in septic shock, abdominal surgery and severe bleeding are associated with AKI. IRI represents a very important condition as it also frequently occurs during kidney, lung and liver transplantation. Ex vivo experiments have shown that EVs exhibit a protective effect on organ oxidative stress and promote tissue integrity in liver and lung perfusion models (91). Several in vivo models of IRI have been used to show the protective effect of EVs derived from different cell sources (bone marrow-derived MSC (40,41), umbilical cord blood-MSC (92), Wharton’s jelly-MSCs (42), renal-cells (43) and endothelial progenitor cells (28). In all these models, EVs seem to act poly-functionally by reducing inflammation and by increasing tubular epithelial cell proliferation. In an IRI model, MSC-EVs also preserved against CKD progression by reducing vascular rarefaction, glomerular and interstitial fibrosis (40). Several papers have consolidated the notion that EVs released from MSC can exert a therapeutic effect in AKI and other renal diseases by transferring their miRNA content (33,88). AKI protection by EVs derived from endothelial progenitor cells was mediated by the transfer of pro-angiogenic miRNAs (28). In fact, the curative and protective effects of EVs were mainly ascribed to the transfer of pro-angiogenic miR-126 and miR-296 to hypoxic resident renal cells. Of interest, Dicer knock-down or specific antagomirs in endothelial progenitor cells (28) as well as Drosha knock-down in MSC (33) blunted the EV biological activities. Moreover, MSC were shown to accelerate recovery and enhance survival in cisplatin-induced AKI (36,37). Likewise, EVs derived from human liver stem cells were shown to favor recovery in a murine model of AKI (34).
In the Thy1.1 glomerulonephritis featuring complement-mediated mesangial cell injury and endothelial cell loss (93), Cantaluppi et al. (29) demonstrated that EVs derived from endothelial progenitor cells inhibit complement-mediated injury. Concerning the translation of EVs into the clinics, in 2014, Kordelas et al. (94) described the use of MSC-derived EVs to treat a patient with conventional therapy-refractory acute graft-versus-host disease. The authors reported no side effect, decrease of symptomatology, and the possibility to reduce steroids. The treatment allowed the patient to remain stable for several months. More recently, Nassar et al. (95) reported the safety of administration of two doses of umbilical cord-blood MSC-EVs, one dose intravenously and one dose intra renal artery, in 20 patients with stage III and IV CKD. No side effects were linked to EV administration. The EV treatment transiently ameliorated the disease progression and improved the renal function and the inflammatory immune reaction.
Fibrosis
The hallmark of CKD is interstitial fibrosis that can be considered a possible target for therapy, since it has been recognized as an independent contributor or predictor of CKD evolution (3). The release of inflammatory mediators, the activation and proliferation of resident fibroblasts, the infiltration of inflammatory/immune cells, the dysregulation of extracellular matrix deposition, and the epithelial to mesenchymal transition are all peculiar features of fibrosis (4).
Kholia et al. (49) studied the role of human liver stem cell-derived EVs in a model of aristolochic acid-induced nephropathy and showed that EVs can prevent interstitial fibrosis by down regulating renal pro-fibrotic genes.
Using a streptozocin-induced type 1 diabetes model, Grange et al. (44) investigated the potential curative effect of repeated i.v. injections of EVs derived from human bone marrow-derived MSC and human liver stem cells. Both the renal function and the glomerular and interstitial fibrosis were significantly improved in stem cell EV-treated animals. Down regulation of several pro-fibrotic genes in renal tissues was correlated with the anti-fibrotic effect of human bone marrow-derived MSC and human liver stem cells. The analysis of the miRNA content of human bone marrow-derived MSC and human liver stem cells showed the presence of some common and specific patterns of miRNAs targeting genes related to fibrosis. Despite some differences, both human bone marrow-derived MSC and human liver stem cells targeted genes coding for well-known mediators of tissue fibrosis such as insulin-like growth factor-1, transforming growth factor-β, platelet derived growth factor receptor and epidermal growth factor receptor. Furthermore, both human bone marrow-derived MSC and human liver stem cells contained miRNAs triggering Collagen I, Snail, and FAS ligand such as miRNA-29a, let-7 family, miRNA-30a, miRNA-24 and miRNA-21. Of interest, human liver stem cell-derived EVs contained high amounts of miR-146a, that inhibits inflammation and fibrosis in CKD and is a potential therapeutic tool for renal fibrosis in a unilateral ureter obstruction model (96,97). MSC-EVs were enriched in let-7 and miR-30 family members that favor renal regeneration (41,98). Urine-purified EVs (45) and MSC-EVs (46) were also shown to have a preventive effect on diabetic nephropathy. Despite the encouraging results indicating that stem cell-derived EVs may attenuate and antagonize the progression of the functional and morphological abnormalities in the streptozotocin-induced model of diabetes, there remains a need for robust mouse models of diabetic nephropathy that mimic key features of advanced human pathology. Nevertheless, preliminary data indicate that MSC-derived EVs may have a role in attenuating type 2 diabetes by reversing peripheral insulin resistance and by reducing beta cell loss (47,48).
EVs as therapeutics: hurdles and perspectives
Several critical issues need to be addressed before EVs may be applied to study protocols in humans. (I) EVs need a classification by the Regulatory Authorities being borderline between advanced therapy medicinal products (ATMPs) and biologics. The classification will relevantly affect the future of EVs as a pharmaceutical product. In favor of the ATMPs is the complex nature of EVs that are small pieces of cells reproducing in a nanoscale magnitude the complexity of the cell of origin. In fact, stem cell derived EVs express surface membranes and intra-cytoplasmic molecules characteristic of the cell of origin including biologically active proteins, lipids, and nucleic acids. Since EVs are a heterogeneous population, the identification of a single biologically relevant component as in biologics would create a major hurdle in the clinical advance of EV-based therapies. (II) Phase 1 studies have to define safety of EV therapy. More specifically, in study protocols with repeated injections of EVs, there will be the need to exclude an antibody response to HLA antigens. (III) In clinical studies based on the pre-clinical models, the amount of EVs to be injected needs a large-scale production. Possible strategies of EV isolation such as standard ultracentrifugation, continuous flow ultracentrifugation or tangential flow filtration need appropriate setups and acceptable recovery as well as maintenance of EV integrity, phenotype characterization and biological potency. (IV) Validated potency assays coherent with the therapeutic application have to be established to evaluate efficacy of produced EVs. (V) Cryopreservation or lyophilization as strategies for storage and related definition of shelf life should be verified. (VI) Finally, the pharmacodynamics, pharmacokinetics and bio-distribution of EVs should be studied after different administration routes (intravenous, intra-arterial, intra-parenchyma injection).
Conclusions
In a time of steep cost increase and with the need to insuring health care for acute and chronic renal disease patients, therapies addressing renal protection need to incorporate several challenging concepts including predictive, preventive and personalized medicine. The crucial role of kidney diseases in affecting whole body balance and other organs’ physiopathology along with the fact that the kidney is the target for immunologic, metabolic and toxic as well as toxic-ischemic causative factors call for a strong commitment of basic science and clinical researchers alike to shape innovative therapies. Type 2 diabetes is a good example on how much would be important to select patients with a higher propensity to develop the disease and progress to CKD. As described, the diabetic microenvironment and even more so the uremic state would be detrimental to the repairing and homeostatic mechanisms. The ability to insure a better glycemic control is very important and may indeed reduce progression to late stages of CKD. Notwithstanding, diabetes may slowly have a negative impact on the kidneys. Stem cells and more specifically their derived EVs hold a promise today. However, we are still far away from solving several fundamental questions related to their final classification by official regulatory agencies, the best choice of the cell origin, the mass production, and their safety and toxicity. As it is usual and right to be so, while basic science is revolutionary in concepts and in suggesting new attractive hypothesis, we are all demanded to seriously accept the task to insure the patients with a truly safe, highly efficient therapy at the timely window of their disease. Based on the increasing evidence of stem cells and their derived EVs in experimental models of renal diseases, new clinical studies will have to confirm the potential of regenerative medicine to enter the therapeutic armamentarium.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ling Lu) for the series “Stem Cell and Clinical Application” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.19). The series “Stem Cell and Clinical Application” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lameire N, Van BW, Vanholder R. Acute renal failure. Lancet 2005;365:417-30. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum J, et al. The ADQI workgroup: Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol 2016;27:687-97. [Crossref] [PubMed]
- Husain-Syed F, McCullough PA, Birk HW, et al. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J Am Coll Cardiol 2015;65:2433-48. [Crossref] [PubMed]
- Park M, Hsu CY, Go AS, et al. Urine Kidney Injury Biomarkers and Risks of Cardiovascular Disease Events and All-Cause Death: The CRIC Study. Clin J Am Soc Nephrol 2017;12:761-71. [Crossref] [PubMed]
- Lameire N, Van Biesen W, Vanholder R, et al. The place of intermittent hemodialysis in the treatment of acute renal failure in the ICU patient. Kidney Int Suppl 1998;66:S110-19. [PubMed]
- Bellomo R, Farmer M, Boyce N. A prospective study of continuous venovenous hemodiafiltration in critically ill patients with acute renal failure. J Intensive Care Med 1995;10:187-92. [Crossref] [PubMed]
- Mehta RL, McDonald B, Gabbai FB, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 2001;60:1154-63. [Crossref] [PubMed]
- Manns B, Doig CJ, Lee H. Cost of acute renal failure requiring dialysis in the intensive care unit: Clinical and resource implications of renal recovery. Crit Care Med 2003;31:449-55. [Crossref] [PubMed]
- Rinkevich Y, Montoro DT, Contreras-Trujillo H, et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 2014;7:1270-83. [Crossref] [PubMed]
- Park J, Shrestha R, Qiu C, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018;360:758-63. [Crossref] [PubMed]
- Nagata M. Podocyte injury and its consequences. Kidney Int 2016;89:1221-30. [Crossref] [PubMed]
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996;334:1448-60. [Crossref] [PubMed]
- Star RA. Treatment of acute renal failure. Kidney Int 1998;54:1817-31. [Crossref] [PubMed]
- Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: Time to consider combination therapy. Semin Nephrol 2000;20:4-19. [PubMed]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis 2002;39:930-6. [Crossref] [PubMed]
- Lameire N. The pathophysiology of acute renal failure. Crit Care Clin 2005;21:197-210. [Crossref] [PubMed]
- Fang W, Wang Z, Li Q, et al. Gpr97 is an important mediator of AKI, and pharmacologic targeting of Gpr97-mediated Sema3A signaling at multiple levels may provide a novel approach for the treatment of AKI. Gpr97 Exacerbates AKI by Mediating Sema3A Signaling. J Am Soc Nephrol 2018;29:1475-89. [Crossref] [PubMed]
- Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat Clin Pract Nephrol 2005;1:22-32. [Crossref] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases: Kidney disease statistics for the United States, 2016. Available online: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed October 1st, 2019.
- United States Renal Data System: 2018 annual data report highlights, 2018. Available online: https://www.usrds.org/adrhighlights.aspx. Accessed October 1st, 2019.
- Chen SF, Chen M. Complement Activation in Progression of Chronic Kidney Disease. Adv Exp Med Biol 2019;1165:423-41. [Crossref] [PubMed]
- Liu BC, Tang TT, Lv LL, et al. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 2018;93:568-79. [Crossref] [PubMed]
- Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med 2004;43:9-17. [Crossref] [PubMed]
- United States Renal Data System: 2018 ADR reference tables. Treatment modalities. Table D.6. Available online: https://www.usrds.org/2018/ref/ESRD_Ref_D_Modality_2018.xlsx
- Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 2007;27:144-52. [Crossref] [PubMed]
- Perkovic V, Jardine MJ, Neal B, et al. CREDENCE Trial Investigators. Canaglifozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306. [Crossref] [PubMed]
- Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect from ischemia-perfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012;82:412-27. [Crossref] [PubMed]
- Cantaluppi V, Medica D, Mannari C, et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial inury in experimental anti-Thy 1.1 glomerulonephritis. Nephrol Dial Transplant 2015;30:410-22. [Crossref] [PubMed]
- Herrera MB, Bussolati B, Bruno S, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 2007;72:430-41. [Crossref] [PubMed]
- Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to renal repair on acute tubular epithelial injury. Int J Mol Med 2004;14:1035-41. [PubMed]
- Hauser PV, De Fazio R, Bruno S, et al. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 2010;177:2011-21. [Crossref] [PubMed]
- Collino F, Bruno S, Incarnato D, et al. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying micrornas. J Am Soc Nephrol 2015;26:2349-60. [Crossref] [PubMed]
- Herrera Sanchez MB, Bruno S, Grange C, et al. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther 2014;5:124. [Crossref] [PubMed]
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004;15:1794-804. [Crossref] [PubMed]
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 2008;26:2075-82. [Crossref] [PubMed]
- Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012;7:e33115. [Crossref] [PubMed]
- Duffield JS, Park KM, Hsiao LL, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005;115:1743-55. [Crossref] [PubMed]
- Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005;289:F31-42. [Crossref] [PubMed]
- Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stemcells protect against ischaemia-reperfusion acute and chronic kidney injury. Nephrol Dial Transplant 2011;26:1474-83. [Crossref] [PubMed]
- Gu D, Zou X, Ju G, et al. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int 2016;2016:2093940.
- Zou X, Zhang G, Cheng Z, et al. Microvesicles derived from Wharton’s jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 2014;5:40-53. [Crossref] [PubMed]
- Ranghino A, Bruno S, Bussolati B, et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 2017;8:24. [Crossref] [PubMed]
- Grange C, Tritta S, Tapparo M, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep 2019;9:4468. [Crossref] [PubMed]
- Jiang ZZ, Liu YM, Niu X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 2016;7:24. [Crossref] [PubMed]
- Nagaishi K, Mizue Y, Chikenji T, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016;6:34842. [Crossref] [PubMed]
- Sun Y, Shi H, Yin S, et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018;12:7613-28. [Crossref] [PubMed]
- Beuzelin D, Kaeffer B. Exosomes and miRNA-Loaded Biomimetic Nanovehicles, a Focus on Their Potentials Preventing Type-2 Diabetes Linked to Metabolic Syndrome. Front Immunol 2018;9:2711. [Crossref] [PubMed]
- Kholia S, Herrera Sanchez MB, Cedrino M, et al. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front Immunol 2018;9:1639. [Crossref] [PubMed]
- Bao YW, Yuan Y, Chen JH. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res 2018;39:72-86. [PubMed]
- Ortiz A, Sanchez-Nino MS, Izquierdo MC. Translational value of animal models of kidney failure. Eur J Pharmacol 2015;759:205-20. [Crossref] [PubMed]
- Quesenberry PJ, Colvin GA, Abedi M, et al. The stem cell continuum. Ann N Y Acad Sci 2005;1044:228-35. [Crossref] [PubMed]
- Guasti L, New SE, Hadjidemetriou I, et al. Plasticity of human adipose-derived stem cells - relevance to tissue repair. Int J Dev Biol 2018;62:431-9. [Crossref] [PubMed]
- Tweedell KS. The adaptability of somatic stem cells: a review. J Stem Cells Regen Med 2017;13:3-13. [PubMed]
- Jiang Y, Jahagirdar BN, Reinhardt R, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41-9. [Crossref] [PubMed]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992;255:1707-10. [Crossref] [PubMed]
- Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science 2000;287:2032-6. [Crossref] [PubMed]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999;96:14482-6. [Crossref] [PubMed]
- Herrera MB, Bruno S, Buttiglieri S, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006;24:2840-50. [Crossref] [PubMed]
- Bussolati B, Bruno S, Grange C, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol 2005;166:545-55. [Crossref] [PubMed]
- Biancone L, Camussi G. Stem cells in 2013: Potential use of stem or progenitor cells for kidney regeneration. Nat Rev Nephrol 2014;10:67-8. [Crossref] [PubMed]
- Tögel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute acute kidney injury. Am J Physiol Renal Physiol 2007;292:F1626-35. [Crossref] [PubMed]
- Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev 2009;18:521-9. [Crossref] [PubMed]
- Ji JF, He BP, Dheen ST, et al. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004;22:415-27. [Crossref] [PubMed]
- Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643-5. [Crossref] [PubMed]
- Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486-96. [Crossref] [PubMed]
- Imberti B, Morigi M, Tomasoni S, et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol 2007;18:2921-8. [Crossref] [PubMed]
- Tögel F, Zhang P, Hu Z, et al. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 2009;13:2109-14. [Crossref] [PubMed]
- Baban B, Liu JY, Payne S. Status of stem cells in diabetic nephropathy: predictive and preventive potentials. EPMA J 2016;7:21. [Crossref] [PubMed]
- Ezquer ME, Ezquer FE, Arango-Rodríguez ML, et al. MSC transplantation: a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res 2012;45:289-96. [Crossref] [PubMed]
- Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis 2012;60:1012-22. [Crossref] [PubMed]
- Swaminathan M, Stafford-Smith M, Chertow GM, et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J Am Soc Nephrol 2018;29:260-7. [Crossref] [PubMed]
- Makhlough A, Shekarchian S, Moghadasali R, et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther 2017;8:116. [Crossref] [PubMed]
- Camilleri ET, Gustafson MP, Dudakovic A, et al. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res Ther 2016;7:107. [Crossref] [PubMed]
- Yun CW, Lee SH. Potential and therapeutic efficacy of cell-based therapy using mesenchymal stem cells for acute/chronic kidney disease. Int J Mol Sci 2019. [Crossref] [PubMed]
- Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019;21:9-17. [Crossref] [PubMed]
- Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016;164:1226-32. [Crossref] [PubMed]
- Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 2011;3:15. [Crossref] [PubMed]
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018;19:213-28. [Crossref] [PubMed]
- Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol 2014;29:116-25. [Crossref] [PubMed]
- Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 2019;8:1648167. [Crossref] [PubMed]
- Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 2006;20:1487-95. [Crossref] [PubMed]
- Ratajczak J, Miekus K, Kucia M, et al. Embrionic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. [Crossref] [PubMed]
- Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440-8. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Lopatina T, Favaro E, Grange C, et al. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci Rep 2018;8:17458. [Crossref] [PubMed]
- Feigerlová E, Battaglia-Hsu SF, Hauet T, et al. Extracellular vesicles as immune mediators in response to kidney injury. Am J Physiol Renal Physiol 2018;314:F9-21. [Crossref] [PubMed]
- Zhang C, Ma P, Zhao Z, et al. miRNA-mRNA regulatory network analysis of mesenchymal stem cell treatment in cisplatin-induced acute kidney injury identifies roles for miR-210/Serpine1 and miR-378/Fos in regulating inflammation. Mol Med Rep 2019;20:1509-22. [PubMed]
- Pomatto MAC, Gai C, Bussolati B, et al. Extracellular vesicles in Renal Pathophysiology. Front Mol Biosci 2017;4:37. [Crossref] [PubMed]
- Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053-67. [Crossref] [PubMed]
- Rigo F, De Stefano N, Navarro-Tableros V, et al. Extracellular vesicles from human liver stem cells reduce injury in an ex vivo normothermic hypoxic rat liver perfusion model. Transplantation 2018;102:e205-10. [Crossref] [PubMed]
- Ju GQ, Cheng J, Zhong L, et al. Microvesicles derived from umbilical cord mesenchymal cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One 2015;10:e0121534. [Crossref] [PubMed]
- Nakamura K, Oka M, Shirai M, et al. Source of reactive oxygen species in anti-Thy1 nephritis. Ren Fail 1998;20:399-405. [Crossref] [PubMed]
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014;28:970-3. [Crossref] [PubMed]
- Nassar W, El-Ansary M, Sabry D, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res 2016;20:21. [Crossref] [PubMed]
- Wang Y, Lu X, He J, et al. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res Ther 2015;6:100-12. [Crossref] [PubMed]
- Morishita Y, Imai T, Yoshizawa H, et al. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis In vivo. Int J Nanomedicine 2015;10:3475-88. [Crossref] [PubMed]
- Park JT, Kato M, Lanting L, et al. Repression of let-7 by transforming growth factor-β1 -induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am J Physiol Renal Physiol 2014;307:F1390-403. [Crossref] [PubMed]


