
Quantum biology in low level light therapy: death of a dogma
“When one looks back over the development of physics, one sees that it can be pictured as a rather steady development with many small steps and superposed on that a number of big jumps… when we have a big jump, it means that something entirely new has to be introduced.”—P.A.M. Dirac [1973]
Introduction
This article highlights a number of recent developments in the field of low level light therapy (LLLT) and points out a number of unresolved problems, thus also trying to predict the direction the evolution may take. At least two things in this approach are arbitrary. “Recent” was chosen to mean “since about 2001”, when the concept of biostimulatory windows (1) was published, to the best of our knowledge the first article pointing to the independence of the basic irradiation parameters light intensity and energy density in LLLT, and defining the intensity threshold. This concept is important for the reproducibility of photobiological effects achieved with red to near infrared (R-NIR) lasers and light emitting diodes (LEDs) and accounts for the success and the failure of the cold laser applications since the pioneering work of Endre Mester in general (2-4), and his last paper published in 1985, in particular (5). As illustrated later, the independence between the two irradiation parameters allows us to understand the fundamental working principle in LLLT and the mechanism of interaction between R-NIR photons and cell. Clearly, as documented by us in detail (6) the root cause of clinically relevant biostimulative effects, e.g., cell viability enhancement, cell proliferation, increase in adenosine triphosphate (ATP) production, cannot be absorption of R-NIR photons by mitochondrial cytochrome c oxidase (COX)—a false dogma propagated since more than 20 years (7-14).
Methods
According to current theory the intrinsic cause for the increase in ATP synthesis in response to irradiation of cells with biostimulatory doses of light is the absorption of R-NIR photons by COX (7-14). This concept is rather ill-defined and unsatisfactory for the following reason. A closer inspection of the measurement which serves today as claim to the concept that COX is the primary absorber for R-NIR photons reveals that it consists of a discrete number of statistically distributed points with exaggerated absorbance values in the R-NIR part of the spectrum (by a factor ≥10) (7), c.f. (15,16). It is not possible to interpret the data (7) in terms of an absorption spectrum. The second work which apparently provides a consistent absorption spectrum for COX in the R-NIR range (8) refers as source to a paper (17) in which the spectrum is absent. These papers (7,8) form the foundation of the widely accepted concept that COX is the primary absorber for R-NIR photons. It is perhaps a coincidence that the same group which claimed the existence of a COX absorption spectrum in Cooper’s paper (17) reported strong absorbance of COX in the R-NIR spectral range presenting the same absorption spectrum which appears in Wong-Riley’s paper (8) in another paper (10). Here too, an absorption spectrum cannot be found in the source reference provided (18). The same pattern repeats itself in Desmet’s study (9) where the authors provide as source reference for the absorption of R-NIR photons by COX (8) in which the reader is guided to the aforementioned study (17) where an absorption spectrum cannot be found. This means, that the currently accepted theory used to explain ATP upregulation by R-NIR light is based on data which cannot be considered as ascertained. If indeed, COX is the principal absorber for the photons which eventually drive the ATP synthase, ATP upregulation must depend on the absorbance profile of COX. Thus, irradiation with 415 nm light [maximum absorbance of both reduced cytochrome c (19) and reduced COX (15) at 415 nm] is expected to result in an ATP output which is superior to that induced by R-NIR light. This expectation receives justification from the currently accepted mechanism of LLLT, in which the absorption of photons by COX is the precondition for an upregulation in ATP levels (7-14). However, contrary to this expectation, irradiation of cells with blue light resulted in a significant drop in intracellular ATP levels concomitant with an increase in intracellular ROS (20,21). Together with the comprehensive analysis presented in Sommer’s study (6) the frustration that had been holding us up from progress in LLLT becomes clear. Despite the logical controversies, the whole climate of opinion was against postulating new models to interpret the interaction of R-NIR photons with oxidatively stressed cells. Too many experimental and clinical results have been published using a wrong model, thus solidifying its further acceptance. How to escape from the limitations of the COX model? One way consists in looking at the new data published on the absorption of COX and in realizing that the absorption in the R-NIR part of the COX spectrum is simply too small for being the root cause for the upregulation of mitochondrial ATP. Indeed, earlier Quirk and Whelan presented results that call into question the COX activity model for R-NIR light (22), thus confirming our earlier work that challenged the COX dogma (23).
Today LLLT has an enormous clinical potential: It started with the successful treatment of non-healing ulcera cruris in diabetic patients and continued with the treatment of dementia, stroke, traumatic brain injury, painful diabetic peripheral neuropathy, depression, retinal disorders, oral mucositis in cancer patients, burns, inflammatory processes, infertility and cosmetic medicine. Besides this short list of exemplary clinical applications there is a growing number of promising results achieved in vitro and animal studies with a potential for translation in clinical trials. In view of these results and the fact that most of them have been realized via a trial an error process defined by the available irradiation equipment, the motivation for a closer examination of the underlying mechanism of action is clear.
Results
Challenging the central dogma in LLLT
Therefore, the assumption that absorption of R-NIR photons by COX plays a central role in the photon-cell interaction and is causal for the aforementioned biostimulative effects cannot be justified on the basis of the available data. Apparently, the LLLT community followed a wrong path for more than two decades. An instructive example for how a dogma could block progress in medical science is that of the postnatal neurogenesis. In 1985 it was generally accepted that the adult human brain is incapable of producing new neurons (24). It took years to declare the death of the dogma (25). Previously, it blocked attempts to use LLLT in neurogenesis—as it is successfully carried out today (26). We note in this context that from the observation that COX is upregulated by R-NIR light one cannot draw the conclusion that COX is the “main photo-acceptor within the effective optical window of LLLT” (27). Actually, the literature on the absorption of R-NIR photons by COX is meagre, in particular at wavelengths ≥1,000 nm. Nevertheless, with the dogma that COX acts as the main acceptor for R-NIR light, it seems natural when it is stated: “However, none of the previous absorption studies have measured photon absorption by CCO at 1,064 nm, and the present study demonstrated a clear effect of this wavelength on CCO upregulation.” (27). The present clinically relevant example, dealing with the oxygenation of hemoglobin in vivo induced by R-NIR light, nicely illustrates the illusory potential of assumptions based on dogma. Lacking a convincing alternative model for the explanation of the observed biostimulative effects, one is tempted to follow the mainstream theory.
If COX is indeed the main mitochondrial photoacceptor, as it is widely believed today (28) then it should be possible to achieve the ATP-powered cell responses which have been realized at higher light intensities (biostimulative effect) also by the irradiation with smaller intensities and extension of the irradiation time, thus anticipating the existence of a dependence between the irradiation parameters light intensity and energy density. This simple expectation, misleading many researchers as well as companies manufacturing LLLT equipment during the early days of LLLT, appeared logical: COX is an enzyme, and as that, the assumed photochemical reaction had to be independent on the intensity of the applied light. A blind alley, as it turned out later. Unfortunately, the physical quantity light intensity was not explicitly addressed in the pioneer work of the Mester group. Lacking information about the importance of the parameter intensity, the cross section of the laser beams was not communicated (5). As discovered and published by the Mester group and confirmed by others (29) the typical dose dependent response of cells to their irradiation with R-NIR light (dose = energy density = intensity × irradiation time) is quantitatively described by an Arndt-Schultz curve (30). Initially, it was established by the use of a powerful ruby laser and 50 mW He-Ne lasers operating at intensities ≥1,000 W/m2.
However, soon after publication of their cornerstone paper (5) the LLLT community realized that it was not possible to reproduce the results published by the Mester group on the grounds of the Arndt-Schultz curve only. In particular, when the light intensities employed were too small the biological effects achieved by the LLLT pioneers with light intensities ≥1,000 W/m2 could not be reproduced, even when the irradiation time was extended and the resulting energy density eventually fell into the empirical 1–4 J/cm2 range. This key observation nourished doubts in the validity of the COX model, subsequently leading to the proposal of a variety of alternative mechanisms. They attempt to surmount the contradictions which prevail in the COX model and to explain the biological effects of R-NIR light without the involvement of COX. However, lacking the apparent conceptual clarity of the COX model, on the one hand, and by avoiding to consider the experimentally confirmed ATP upregulation following exposure to biostimulatory levels of R-NIR light (31,32), on the other hand, even the most popular alternative models (33,34) could not compete with the mainstream theory. In fact, not one of the alternative theories put forward so far gained the acceptance of the COX hypothesis, as can be checked, e.g., in Google Scholar, and more importantly, they show limitation in both agreement with experiment and predictive capability. However, it is not within the scope of this short paper to address this issue. Our focus is exclusively the COX hypothesis. From the literature published so far it is now evident that it must be something totally different from the COX complex that is instrumental in converting light energy into ATP.
Precondition for biostimulative light effect: oxidative stress
It is instructive at this point to recall another important observation of the Mester group: They pointed out that the beneficial effects of LLLT, e.g., accelerated cell proliferation, angiogenesis, collages synthesis, swelling of mitochondria, etc. are solely observable in oxidatively stressed systems, i.e., cells in vitro and tissues in vivo. Notably, at the beginning of the treatment changes in the clinical picture are typically absent. So, departure from normal homeostatic states on the cellular level is one precondition for successful results in LLLT. Assuming a causal dependence between the treatment of oxidatively stressed systems with R-NIR light and the normalization of mitochondrial ATP levels, including but not limited to, clinically relevant cellular responses such as accelerated proliferation, viability extension, enhanced collagen and elastin synthesis, stress survival, gamma ray protection, angiogenesis and autophagocytosis, we are indicated that R-NIR light is instrumental in bringing something that is out of balance back to normal, i.e., restoring normal homeostasis. Neither the popular COX hypothesis nor the alternative models are capable to provide a satisfactory explanation to this scenario, which could be summarized in simple words: If oxidative stress is quantitatively reflected by an increase in mitochondrial reactive oxygen species (ROS) and if ROS is inhibiting cellular function including the synthesis of mitochondrial ATP then the beneficial effect of R-NIR light must consist in counteracting the inhibitory effect of ROS. This relationship leads us to the next question and from there to the solution of the problem: How can ROS be involved in the inhibition of ATP synthesis? In order to answer the question we recall that ATP is synthesized by the mitochondrial rotary motor called ATP synthase. Anchored in the mitochondrial membrane it rotates with 9,000 rpm in a predominantly hydrophilic milieu embedded in an aqueous environment. Previous laboratory experiments demonstrated that the viscosity of the nanoscopic water layers (ca. 2–3 monolayers) attached to hydrophilic surfaces is extremely high—several magnitudes above that of bulk water—reaching values comparable to molasses. Moreover, this interfacial viscosity increases both with confinement and hydrophilicity. Considering that ROS consists in most cases of an oxygen with a negative charge, a combination known to increase the hydrophilic character of surfaces, it becomes clear that extended ROS bombardments will inevitably increase the hydrophilicity within and around the mitochondrial nanomotor. The effect of the associated viscosity increase on the nanomotor can only be rotational drag, thereby providing a plausible explanation to the drop in ATP production in response to oxidative stress. Excluding absorption by COX as root cause for R-NIR light-induced synthesis of mitochondrial ATP we are quickly led to the need for a paradigm shift in LLLT: The simple physical process involving a reduction in the viscosity of the fraction of mitochondrial bound water (35) recommends itself as a target for the photons. This picture is in harmony with the fact that mitochondrial ATP synthesis can be triggered by various wavelengths of light. We only have to omit those which are strongly absorbed by COX.
Derivation of the Arndt-Schultz behavior in LLLT
The cytotoxic and pro-inflammatory effect of extracellular ATP are well documented (36-38). The capacity of the ATP molecules to cross the plasma membrane, for instance, via metabolic processes or cell regulated release of excess ATP following overexposure of cells to R-NIR light and rapid release of excess intracellular ATP into the extracellular space (39)—after filling the intracellular reservoirs—has also been described. Today, there is an abundance of evidence which indicates that ATP is released as well as taken up by cells (40,41).
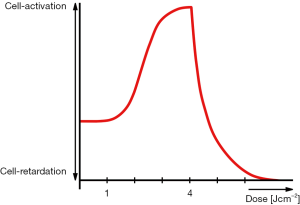
With the picture of a cloud of ATP molecules accumulating in the space proximal to the plasma membrane, we are offered an intuitive explanation to the descending part of the bell-shaped Arndt-Schultz curve (30). The synergistic interplay with an intracellular enrichment of ROS justifies the assumption that it will exert an instant cytotoxic effect on the affected cells and tissues. The envisaged scenario is, in principle, similar to that of sperm cells in vitro stewing in their own ROS at the bottom of the polystyrene Petri dish—as opposed to the chemically and biologically inert diamond surface (42), a powerful picture eloquently portrayed by Michael Price in Science (43). Furthermore, ROS bombardments originating from an overexposure to R-NIR light was predicted to additionally contribute to a reduction in the output capacity of the ATP synthase via rotational drag (44). Concurrently, the ascending part of the Arndt-Schultz curve is straightforwardly derived from the instant drop in the viscosity of the nanoscopic interfacial water layers [mitochondrial bound water (35)] in response to their irradiation with moderately intense R-NIR light. Last but not least, the maximum of the Arndt-Schultz curve (optimal dose in LLLT) can be beautifully explained on the basis of equilibrium between increase in light-induced synthesis of mitochondrial ATP and the ATP consumption of the cell under conditions normal homeostasis (Figure 1).
Discussion
Confusing nomenclature
A further point, closely related to the aforementioned aspects concerns the current trend to deviate from the original nomenclature (biostimulation) coined by Endre Mester and instead to use the novel term photobiomodulation—a phenomenon which instead of bringing clarity into the field causes confusion and leads us astray, as reflected by a recent editorial, displaying the chaos in the field (45). Any combination constructed with the word biomodulation means deviation from logic. To see this, we recall the conventional interpretation of “modulation”. It involves the process of varying the properties of a high-frequency periodic waveform. The term would make sense if it would refer to the modulation of the light itself. And viewed from the Arndt-Schultz perspective, the biomodulation concept totally fails when it is used, e.g., for over-stimulation (cell retardation in response to doses above a certain maximum). Can we over-modulate a cell? The term over-modulate is rarely used in the scientific literature, and if, then mostly in a relationship to music or electronic devices. A quick PubMed search illustrates that the number of researchers using the novel term photobiomodulation (PBM), or as replacement for LLLT photobiomodulation therapy (PBMT), is considerably smaller than that of those using the term LLLT. Further, the acronyms PBM and PBMT cover a large variety of topics which are totally different from the biomedical use of light, whereas the catchy acronym LLLT is unmistakably assigned to light therapy with a total of 6,068 hits in PubMed as opposed to PBMT with no more than 118 hits at the time of the writing. As can be easily verified, a considerable fraction of the acronym PBMT occurring in PubMed relate to subjects which are quite foreign to biological applications of light, and stand, for instance, for paratubal borderline mucinous tumor, pediatric blood and marrow transplant, parent behavioral management training, PPI bismuth metronidazole tetracycline. The same trend with even more variations is reflected in Google Scholar. Addressing this mismatch is not only of academic character. By splitting the nomenclature of the entire cold laser field into two parts, the traditional literature using the term LLLT and the novel fragment using PBMT, literature searches are becoming more difficult and novices will certainly ask: what is the difference?
Besides providing ATP for cellular functions, mitochondria play a prominent role in the social defense against environmental stresses causing excess ROS production—the coup de grâce for the affected cell. Because the hypothesis that the absorption of R-NIR photons by mitochondrial COX is a precondition for ATP synthesis cannot be maintained, when based on the published literature, we are forced to turn our attention towards alternative theories, otherwise we are left with a methodological vacuum concerning the photon-cell interaction mechanism. Without a scientifically sound mechanism the entire LLLT field would reduce to empiricism. Hence, the need for a valid mechanism accounting for the photon-cell interaction. In lack of such a basic construct, the impressive biomedical results achieved so far by the scientific community could easily be subjected to strong criticism. In other words, after the proposal of the COX absorber theory, researchers were grateful to place their work upon a solid scientific platform, on the one hand, and editors of high impact factor journals were more and more willing to accept papers related to LLLT, on the other hand. As a result, the field of LLLT begun to flourish, gaining wide acceptance with the result that the number of clinical and experimental papers reporting on LLLT applications started to grow exponentially, as it is the case today. Actually, the inertia of the amount of papers published to date on LLLT related work, with medically captivating results, safeguards the continuation of the development, however, as long as a wrong map is used, in the wrong direction and without much progress.
Don’t forget COX
In a recent paper Maik Hüttemann et al. presented surprising results which hold the promise to initiate another big jump into the conceptual framework of LLLT. After a systematic scan with frequencies between 700 and 1,000 nm the group reported that certain frequencies (750 and 950 nm) reduced the activity of COX (via inhibition of the reaction of cytochrome c and COX) and limited ROS generation (46,47). This is an unexpected discovery which has already practical applications of maximum clinical relevance, as reflected by the recovery of pigs 13.5 minutes post cardiac arrest, resuscitation and irradiation of their foreheads with LED light (Maik Hüttemann, personal communication). It will be interesting how these novel results and their interpretation by us will help to overcome the conflict between current theoretical models in LLLT.
One further reason to discard the concept that COX is the central absorber for R-NIR photons, and thereby the key determinant in light-induced ATP upregulation, is derived from the results of a recent experimental paper of Shimada et al. In it, the authors used high resolution imaging methods to study details of the interaction between cytochrome c and COX with emphasis on the transport of electrons between the two enzymes. One of most striking findings in the work comprises the description of 3 monolayers of H2O at the interface between the enzymes (48). From the predominantly hydrophilic nature of the proximal enzyme sites, and in concert with previous laboratory experiments exploring the effect of R-NIR light on nanoscopic interfacial water layers attached to different surfaces, it is realistic to expect that exposure of the enzyme complex to biostimulatory intensities of R-NIR light results in an instant drop in the viscosity of the nanoscopic interfacial water layer established by H2O monolayers (44), complemented by a spatial separation (volume expansion) between the proximal enzyme sites (49). It is also possible that the volume expansion encourages the influx of additional H2O molecules into the space between the irradiated enzymes. This simply means that the pathway of electrons crossing between the proximal sites of electronically interacting enzymes will be longer when compared to non-irradiated enzyme systems. In accordance with the physical picture depicted here and the dimension of the interacting components it is reasonable to interpret the nanolayer of H2O molecules separating the two enzymes in terms of a quantum mechanical barrier. This naturally leads to the question: what is the effect of the light-induced change in the H2O nanolayer on the transport of electrons? For electrons crossing between the proximal enzyme sites separated by a nanolayer of H2O molecules quantum mechanical tunneling recommends itself as the only possible mechanism of transfer. Indeed, quantum mechanical tunneling is recognized today as a viable route to enhance reaction rates in enzymes. The history providing experimental evidence for electron tunneling in a biosystem started with studying the rate of oxidation of cytochrome following absorption of a light pulse from a ruby laser in a photosynthetic bacterium (50,51). Because of their implications in biological systems in general, and electronic communication between enzymes, in particular, electron tunneling through 1–4 monolayers of H2O has been recently the focus of extensive research efforts (52-54).
Establishing contact with the results of Shimada et al. (48) it becomes clear that by reinforcing the barrier property of the nanoscopic interfacial water layers, biostimulatory levels of R-NIR light are more likely to hinder electron passage between mitochondrial cytochrome c and COX, than to facilitate it. Earlier, we showed that biostimulatory intensities of R-NIR light—for which bulk water is practically transparent—possess the capacity to alter the molecular structure of nanoscopic interfacial water layers by a mechanism identified as collective hydrogen bond excitation (49,55). Apparently the degree of the excitation is different for different wavelengths of R-NIR light (56). Typically, light excitation and accompanying structural changes affect 2–3 monolayers of water (56). Translation of these results to the effect of 750 and 950 nm light on the transfer of electrons between cytochrome c and COX, and the connected decrease in the release of mitochondrial ROS, as reported by the Hüttemann group (46,47), is straightforward. From our own findings (44,49,56), together with the observations of Shimada et al. (48), we propose that the beneficial effect is due to a perturbation of the normal electron transfer between cytochrome c and COX by 750 and 950 nm light via maximum excitation in the organization of the nanoscopic interfacial water layer prevailing at the interface, and as a result, a longer tunneling pathway for electrons. As explained in the following section, the number of electrons tunneling through a quantum barrier decreases exponentially with the width of the barrier. Even a minimal increase in the width of the barrier can result in an interruption of the electron flow.
In the past two decades, the distance dependence of water-mediated electron transfer reaction rates between biomolecules has become the focus of intensive experimental and theoretical investigation. As an example, it is instructive to consider a number of electrons all of which have the kinetic energy E and tunnel across the interfacial water layer barrier between two enzymes. The electrons are incident from one side on a potential barrier of height U-E, where E<U. The transmission probability T for an electron to pass through a barrier of width L is equal to
|
| [1] |
|
| [2] |
with m and ħ standing for the mass of the electron and the Planck constant h divided by 2π, respectively (57). From Eq. [1] and [2] we can see that T decreases exponentially with the width of the barrier. From the square root in Eq. [2] it is evident that T is more sensitive to the width of the barrier that to its height. Translation of the trend described by Eq. [1] to the constellation at the interface between cytochrome c and COX (48) allows us envisage that even a minimal increase in spatial separation between the biomolecules due to the effect of R-NIR on the interfacial water layers between the enzymes will inevitably cause a substantial drop in the electron flow between them. The result, at the moment only qualitative, is a strong argument in favor of the postulate that COX is not the main absorber of biostimulative intensities of R-NIR light and simultaneously provides a reasonable explanation to the findings of the Hüttemann group (46,47).
Biological field-effect transistor (FET) in mitochondria
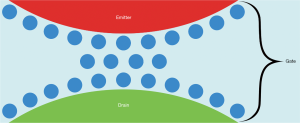
From the quantum mechanical perspective one picture comes to mind immediately: With the enzymes cytochrome c and COX, the nanoscopic interfacial water layer sandwiched between them and the R-NIR photons controlling the tunneling of electrons across the interfacial water barrier, the analogy with a FET is obvious. Considering the arrangement of the interacting elements of the coupled mitochondrial enzymes and the functional interplay between them allows us to identify the constituents of a conventional FET with: cytochrome c acting as emitter, COX acting as drain and the nanoscopic interfacial water layer between the two enzymes acting as gate (Figure 2), where the terms emitter, drain and gate are customary for FETs. In contrast to the working principle of a conventional FET with a semiconductor gate, in which the gate voltage controls the channel current, the electrons tunnel in the biological FET through the interfacial water layer, and the tunneling is controlled by light. At the moment, we only see the possibility to use light to attenuate the tunneling of electrons. This does not mean, however, that the opposite effect (tunneling amplification by light) is impossible. Guided by the powerful picture of the biological FET somebody may be able discover specific irradiation parameters (wavelength of light and/or light intensity and/or pulse frequency) which may facilitate the tunneling of electrons through the nanoscopic interfacial water layer between cytochrome c and COX. It is not clear whether the consequence will be constructive, a blip or a catastrophe.
An attempt at a comprehensive theory and identification of the intrinsic cause of the biostimulative effect of R-NIR light in general, and the upregulation of ATP in oxidatively stressed cells, in particular, has been made. In this way, a new scheme based on quantum biology, which is more suitable for the description of phenomena observed in LLLT has been introduced. The range of phenomena correctly described by the new scheme, as well as its superior predictive capability when compared to the classical LLLT theory, in which COX serves as the main absorber for R-NIR light, shows the necessity for a departure from the current dogma. In addition, the quantum biological approach has the advantage that we can use the tools of quantum mechanics to calculate various possible photon-cell interaction modalities, an aspect, which is completely missing within the classical scheme. The calculations are not easy, but with help from the theoretical site we may hope that by exploiting the predictive capability of the new model, we will eventually be able to leave the long path of trial and error, where the response of biological systems to their stimulation with R-NIR photons virtually reduced to coincidence, including the molecular target, wavelength of photons, intensity and energy density of the light. With knowledge of the relevant molecular targets and their reaction to R-NIR photons we are now in the position to start to systematically use LLLT with optimal treatment parameters in nanomedicine, cell based therapies and personalized medicine.
Conclusions
In view of the addressed conceptual incoherencies, it is not easy to predict the direction the evolution in LLLT may take. Many of the current models used to interpret the photon-cell interaction are not reliable at all, even though many people are working on them and their work is sometimes done in very great detail. Previously, Passarella et al. reported that 632.8 nm laser light changed the energy metabolism in mitochondria irradiated in vitro, and elevated ATP levels. The authors suggested that the extra ATP synthesis is directly produced by a laser-induced extra proton-motive force (31). Unfortunately, the scientific community did not pick up the radical idea. Instead they preferred the COX model (28). From the perspective of our own findings with regard to the contradictions in the currently accepted COX model, the result of the data presented by Lima et al., showing that cell proliferation following irradiation with 660 nm light did not require the presence of COX (58), are not surprising. Although the classical idea that COX is the principal absorber for R-NIR photons meets with a considerable amount of success in the literature, reflected by the more than 2,000 hits in Google Scholar, it fails completely on certain fundamental points, mentioned in our paper. This calls into question most of the theoretical principles which had been formerly regarded as permanently established in LLLT. After having challenged the old theory and the exposure of its dogmatic nature one is tempted to expect rapid progress in both theoretical and clinical development of LLLT, though not without stout resistance occasionally on the part of some valiant upholder of the established theory. The scientifically exciting data presented by Lima et al. (58) strongly challenge the established mechanism of action in LLLT and have the potential to transform the theoretical architecture of the LLLT field; the ignorant interpretation (59) of this important article illustrates the prevalent atmosphere when it comes to truly innovative results in LLLT.
Now, what should we do in the present situation? We feel that we have to insist on the validity of the proton-motive force picture and work on it until we arrive to a fundamentally correct model accounting for the entire spectrum of the interaction modalities between photons and cells. Whereas the discovery of the Hüttemann group nicely shows that certain wavelengths of R-NIR light are instrumental in blocking the cooperative effect of cytochrome c with COX, it cannot provide an explanation for the increase in ATP upon irradiation of cells with R-NIR light. The effect of R-NIR light on the viscosity of mitochondrial bound water fits well into the new fundamental model. To further advance, we only have to see the big picture presented in (6): a unified model for the interaction of R-NIR photons with both mitochondria and cells based on perturbations caused by the photons, instead of an ill-defined absorption processes by COX.
Acknowledgments
We dedicate this work to Julius Edgar Lilienfeld, inventor of the field-effect transistor, on the occasion of his 137th birthday.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.159). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was not required. Biological experiments were not necessary to design the model and perform the analysis of the data presented.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sommer AP, Pinheiro AL, Mester AR, et al. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg 2001;19:29-33. [Crossref] [PubMed]
- Mester E.. The use of the laser beam in therapy. Orv Hetil 1966;107:1012-6. [PubMed]
- Mester E, Spiry T, Szende B, et al. Effect of laser rays on wound healing. Am J Surg 1971;122:532-5. [Crossref] [PubMed]
- Mester E. Über die stimulierende Wirkung der Laserstrahlung auf die Wundheilung. In: Der Laser: Grundlagen und Klinische Anwendungen. Dienstl K, Fischer PL. editors. Berlin, Heidelberg, New York: Springer 1981:109-18.
- Mester E, Mester AF, Mester A. The biomedical effect of laser application. Lasers Surg Med 1985;5:31-39. [Crossref] [PubMed]
- Sommer AP. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: the principles of low-level light therapy. Ann Transl Med 2019;7:S13. [Crossref] [PubMed]
- Karu T.. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 1999;49:1-17. [Crossref] [PubMed]
- Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761-71. [Crossref] [PubMed]
- Desmet KD, Paz DA, Corry JJ, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg 2006;24:121-8. [Crossref] [PubMed]
- Whelan H, Desmet K, Buchmann E, et al. Harnessing the cell's own ability to repair and prevent neurodegenerative disease. SPIE Newsroom 2008;2008:1-3.
- Karu TI. Cellular and Molecular Mechanisms of Photobiomodulation (Low-Power Laser Therapy). IEEE J Sel Top Quantum Electron 2014;20:7000306. [Crossref]
- Hennessy M, Hamblin MR. Photobiomodulation and the brain: a new paradigm. J Opt 2017;19:013003. [Crossref] [PubMed]
- Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol 2018;94:199-212. [Crossref] [PubMed]
- Paiva ACM, da Fonseca AS. Could adverse effects and complications of selective laser trabeculoplasty be decreased by low-power laser therapy? Int Ophthalmol 2019;39:243-57. [Crossref] [PubMed]
- Mason MG, Nicholls P, Cooper CE. Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non invasive in vivo monitoring of tissues. Biochim Biophys Acta 2014;1837:1882-91.
- Malatesta F, Antonini G, Sarti P, et al. Structure and function of a molecular machine: cytochrome c oxidase. Biophys Chem 1995;54:1-33. [Crossref] [PubMed]
- Cooper CE, Springett R. Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy. Philos Trans R Soc Lond B Biol Sci 1997;352:669-76. [Crossref] [PubMed]
- Beauvoit B, Evans SM, Jenkins TW, et al. Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal Biochem 1995;226:167-74. [Crossref] [PubMed]
- Koch HG, Schneider D. Folding, assembly, and stability of transmembrane cytochromes. Current Chem Biol 2007;1:59-74.
- Wang Y, Huang YY, Wang Y, et al. Red (660nm) or near-infrared (810nm) photobiomodulation stimulates, while blue (415nm), green (540nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep 2017;7:7781. [Crossref] [PubMed]
- Kushibiki T, Hirasawa T, Okawa S, et al. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg 2013;31:95-104. [Crossref] [PubMed]
- Quirk BJ, Whelan HT. Effect of red-to-near infrared light on the reaction of isolated cytochrome c oxidase with cytochrome c. Photomed Laser Surg 2016;34:631-7. [Crossref] [PubMed]
- Sommer AP. A mechanism for ultrasound/light-induced biostimulation. Ann Transl Med 2015;3:291. [PubMed]
- Rakic P.. DNA synthesis and cell division in the adult primate brain. Ann N Y Acad Sci 1985;457:193-211. [Crossref] [PubMed]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 2000;1:67-73. [Crossref] [PubMed]
- Yang L, Tucker D, Dong Y, et al. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp Neurol 2018;299:86-96. [Crossref] [PubMed]
- Wang X, Tian F, Soni SS, et al. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 2016;6:30540. [Crossref] [PubMed]
- Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B 2014;140:344-58. [Crossref] [PubMed]
- Ohshiro T. Low Level Laser Therapy: A Practical Introduction. New York: John Wiley and Sons, 1988:30.
- Oshiro T.. Light and life: A review of low reactive-level laser therapy, following 13 years’ experience in over 12000 patients. Laser Ther 1993;5:5-22.
- Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 1984;175:95-9. [Crossref] [PubMed]
- Karu T., Pyatibrat L., Kalendo G.. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 1995;27:219-23. [Crossref] [PubMed]
- Lubart R, Eichler M, Lavi R, et al. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 2005;23:3-9. [Crossref] [PubMed]
- Lubart R, Lavi R, Friedmann H, et al. Photochemistry and photobiology of light absorption by living cells. Photomed Laser Surg 2006;24:179-85. [Crossref] [PubMed]
- Ford RC, Ruffle SV, Ramirez-Cuesta AJ, et al. Inelastic incoherent neutron scattering measurements of intact cells and tissues and detection of interfacial water. J Am Chem Soc 2004;126:4682-8. [Crossref] [PubMed]
- Cauwels A, Rogge E, Vandendriessche B, et al. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 2014;5:e1102. [Crossref] [PubMed]
- Volonté C, Amadio S, Cavaliere F, et al. Extracellular ATP and neurodegeneration. Curr Drug Targets CNS Neurol Disord 2003;2:403-12. [Crossref] [PubMed]
- Murgia M, Pizzo P, Steinberg TH, et al. Characterization of the cytotoxic effect of extracellular ATP in J774 mouse macrophages. Biochem J 1992;288:897-901. [Crossref] [PubMed]
- Wang L, Hu L, Grygorczyk R, et al. Modulation of extracellular ATP content of mast cells and DRG neurons by irradiation: studies on underlying mechanism of low-level-laser therapy. Mediators Inflamm 2015;2015:630361.
- Chaudry IH. Does ATP cross the cell plasma membrane. Yale J Biol Med 1982;55:1-10. [PubMed]
- Dahl G.. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 2015. [Crossref] [PubMed]
- Sommer AP, Jaganathan S, Maduro MR, et al. Genesis on diamonds II: contact with diamond enhances human sperm performance by 300%. Ann Transl Med 2016;4:407. [Crossref] [PubMed]
- Price M. Diamond dishes could boost IVF success rates. Available online: https://www.sciencemag.org/news/2017/04/diamond-dishes-could-boost-ivf-success-rates
- Sommer AP, Haddad MKh, Fecht HJ. Light effect on water viscosity: Implication for ATP biosynthesis. Sci Rep 2015;5:12029. [Crossref] [PubMed]
- Anders JJ, Arany PR, Baxter GD, et al. Light-Emitting Diode Therapy and Low-Level Light Therapy Are Photobiomodulation Therapy. Photobiomodul Photomed Laser Surg 2019;37:63-5. [Crossref] [PubMed]
- Strubakos CD, Malik M, Wider JM, et al. Non-invasive treatment with near-infrared light: A novel mechanisms-based strategy that evokes sustained reduction in brain injury after stroke. J Cereb Blood Flow Metab 2020;40:833-44. [Crossref] [PubMed]
- Sanderson TH, Wider JM, Lee I, et al. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain schemia/reperfusion injury. Sci Rep 2018;8:3481. [Crossref] [PubMed]
- Shimada S, Shinzawa-Itoh K, Baba J, et al. Complex structure of cytochrome c-cytochrome c oxidase reveals a novel protein-protein interaction mode. EMBO J 2017;36:291-300. [Crossref] [PubMed]
- Sommer AP, Hodeck KF, Zhu D, et al. Breathing Volume into Interfacial Water with Laser Light. J Phys Chem Lett 2011;2:562-5. [Crossref]
- DeVault D, Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J 1966;6:825-47. [PubMed]
- DeVault D, Parkes JH, Chance B. Electron tunnelling in cytochromes. Nature 1967;215:642-4. [Crossref] [PubMed]
- Benjamin I, Evans D, Nitzan A. Electron tunneling through water layers: Effect of layer structure and thickness. J Chem Phys 1997;106:6647. [Crossref]
- Hahn JR, Hong YA, Kang H. Electron tunneling across an interfacial water layer inside an STM junction: tunneling distance, barrier height and water polarization effect. Applied Physics A 1998;66:S467-72. [Crossref]
- Lin J, Balabin IA, Beratan DN. The nature of aqueous tunneling pathways between electron-transfer proteins. Science 2005;310:1311-3. [Crossref] [PubMed]
- Walski T, Dąbrowska K, Drohomirecka A, et al. The effect of red-to-near-infrared (R/NIR) irradiation on inflammatory processes. Int J Radiat Biol 2019;95:1326-36. [Crossref] [PubMed]
- Sommer AP, Zhu D, Försterling HD, et al. Crystalline Water at Room Temperature − Under Water and in Air. Cryst Growth Des 2008;8:2620-2. [Crossref]
- Beiser A. Concepts of Modern Physics, 5th ed. McGraw-Hill, 1995:179-81.
- Lima PLV, Pereira CV, Nissanka N, et al. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J Photochem Photobiol B 2019;194:71-5. [Crossref] [PubMed]
- Hamblin MR. Photobiomodulation for Alzheimer's disease: Has the light dawned? Photonics 2019. [Crossref] [PubMed]


