Thoracic sympathetic nerve reconstruction for compensatory hyperhidrosis: the Melbourne technique
Introduction
Hyperhidrosis is a condition characterized by increased production of sweat disproportionate to the amount required to adapt to change in environmental conditions or thermoregulatory needs (1). It has equal prevalence among both men and women affecting 2.9% of the general population (2). Although hyperhidrosis is a benign condition, it often causes embarrassment resulting in significant psychosocial morbidity to the patient (2).
Hyperhidrosis can be generalized or focal. Generalized hyperhidrosis affects the entire body and is usually secondary in nature. Focal hyperhidrosis (i.e., primary hyperhidrosis) is usually idiopathic and involves specific body sites, most commonly the axillae, palms, soles and face (3,4). It is postulated that over-activity of the sympathetic nervous system in response to environmental or emotional stimuli is the main cause of primary hyperhidrosis (1), hence the rationale for the sympathectomy in severe cases that are refractory to non-surgical treatments.
Endoscopic thoracic sympathectomy (ETS) is a definitive surgical treatment for primary hyperhidrosis which evolved since the 1950s (5). ETS involves electrocautery or clipping of the thoracic sympathetic chain intended to interrupt the nerve tracks and nodes that transmit signals to the sweat glands. The efficacy and the benefit of ETS are well documented (1). Despite the high success rate and low morbidity, it is often associated with a significant long-term complication, namely compensatory hyperhidrosis (CH), which occurs as late as 6 months postoperatively (6), in other areas of the body, notably the trunk, and lower limbs.
The true incidence of CH following ETS is difficult to attain due to lack of standardization in definitions and anatomical variations (7). Lai et al. introduced the severity scale of CH (8), however, its applicability is questionable since assessment is broadly subjective without a standardized objective measuring tool (7). Nevertheless, the incidence of postoperative CH ranges from 30% to 100% (9-13), and that of severe CH is as high as 43% (8).
The exact mechanism of CH is unknown but several theories include: a relative reduction of effective body surface area for evaporative heat loss resulting in higher sweat production in areas with intact sudomotor function (14) or increased thermosensitivity and altered reflex response of the hypothalamic thermal controller post-ETS (9).
It is difficult to treat CH. As a matter of fact various surgical techniques and protocols of ETS are continually reformed to minimize occurrence of CH but no curative treatment exists to date. Consequently, reversal surgeries for CH have been attempted with variable yet promising results (15,16).
To date, there have been no reports of reversal surgery for CH using autogenous vein graft as a nerve conduit to reconstruct thoracic sympathetic nerves. Therefore we present this novel technique, which was performed in two patients, and their long-term outcome.
The Melbourne technique
A novel surgical technique, which was developed by Australian surgeons in Melbourne, was employed in these two cases. This technique requires positioning the patient in a semi-seated position with arms in abduction (modified Fowler position). The procedure was undertaken with general anaesthesia using double-lumen endotracheal tube. In both cases, the procedure was performed on the right side only. Three ports (1 mm × 5 mm, 2 mm × 3.5 mm) were accessed over previous scars, along anterior axillary line and mid-clavicular line at the level of the fifth intercostal space, and mid-axillary line at level of third intercostal space. A controlled pneumothorax was achieved with CO2 and pleural space was inspected with 3.3 mm 30° telescope (KARL STORZ, GmbH&Co. KG, Tuttlingen, Germany). Careful pleural adhesiolysis and pleural windows were made using hook diathermy and scissors. The T2 sympathetic trunk was identified at the neck of 2nd rib and its distal segment over 3rd rib. Neither case had titanium clips in situ from previous surgery; however, if present they would have been removed. In case 1, neuromas formed at the proximal and distal ends of T2 sympathetic trunk were excised leaving a defect of approximately 1 cm (Figure 1). In case 2, T2-3 sympathetic trunk neuromas were carefully neurolysed and excised, resulting in a 1 cm defect.
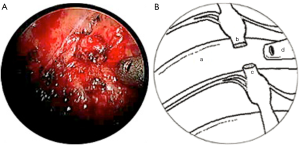
In both cases, a segment of superficial vein (5 cm in length) was harvested from the left forearm. The vein was flushed with heparinized saline and its orientation reversed. The harvested veins were resized to the length of the defect, and both ends were splayed open to accommodate the nerve trunk stumps. End-to-end coaptation was secured in position with a fine layer of fibrin sealant (TISSEEL™, Baxter International Inc., USA) (Figures 2,3,4).
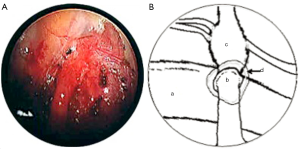
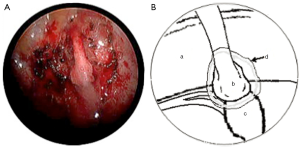
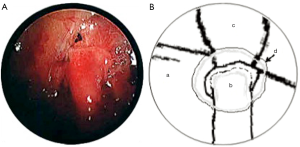
Finally, the lung was re-expanded and an intercostal catheter placed to remain in situ for 24 hours until complete resolution of the pneumothorax was confirmed on a postoperative chest X-ray. The average length of the operation was 120 minutes.
Case 1
Patient A, a right-hand-dominant 58-year-old Caucasian male, underwent ETS in December 2002 by clipping T2 and T3 sympathetic trunks bilaterally for severe primary facial hyperhidrosis and rubor facialis affecting his quality of life (QoL). He noticed significant reductions in facial sweating and blushing, attaining a hyperhidrosis disease severity scale (HDSS) (17) score of 1 from the initial 4. However, at 5 months post-ETS, he developed symptoms of CH of his trunk and axillae. At 6 months and 24 months post-ETS, he underwent removal of the right T2 clip, and the right T3 clip respectively. Despite the removal of the titanium clips and a trial of Ditropan® (Oxybutynin chloride), he continued to experience excessive sweating on his back and axillae.
At approximately 5 years post-ETS, he underwent reversal surgery using the Melbourne Technique. A 1 cm segment of scarred right thoracic sympathetic chain was excised which was later reported as nerve with surrounding fibrous stroma.
Within 6 months post-reversal surgery, he noticed a return of perioral sweating, which was reportedly bearable. Simultaneously, he observed mild to moderate reduction in sweating on his back and axillae to a tolerable level.
At 5 years post-reversal surgery, he continued to experience mild to moderate sweating on his back, however, to a lesser degree following reversal surgery. The patient’s dermatology life quality index (DLQI) score improved from 12 (pre-reversal surgery) to 6 (i.e., moderate effect on patient’s life), and QoL questionnaire (18) at 5 years post-reversal surgery was slightly better.
Case 2
Patient B, a right-hand-dominant 49-year-old Caucasian male, underwent bilateral ETS in 2001 for severe primary facial hyperhidrosis affecting his QoL. Bilateral T2 sympathetic trunks were excised with electrocautery. He noticed significant reduction in facial sweating thereafter, reflected by improvement of HDSS score from 4 to 1. However, after 3 months following ETS, he developed compensatory sweating involving trunk and axillae. Several non-surgical interventions were trialed without a definitive benefit which included: CT-guided lumbar sympathetic plexus block; Pro-Banthine® (Propantheline bromide); Ditropan® (oxybutynin chloride); and drysol (aluminium chloride hexahydrate) spray.
At approximately 8 years after ETS, the patient underwent reversal surgery using the Melbourne technique. Axillary hyperhidrosis improved within three months following the reversal surgery. At four years post-reversal surgery, trunk hyperhidrosis remained moderately persistent, however, patient noticed a gradual return of noticeable but tolerable perioral sweating. The patient’s DLQI score improved from 18 (pre-reversal surgery) to 15 (i.e., very large effect on patient’s life), and QoL questionnaire (18) at four years after the reversal surgery was about the same.
Discussion
The reversal surgery following ETS is usually reserved for those with severe CH, refractory to medical treatment. In those who underwent thoracic sympathotomy with clipping method, subsequent removal of clips is also considered a form of reversal. In our first case, removal of the clips from both T2 and T3 (although from right side only) did not alleviate symptoms.
The basic aim of the reversal surgery is to reduce the severity of CH by reconstructing the sympathetic trunk that was once divided, which also includes excising iatrogenic neuromas. The primary approach is endoscopic through previous thoracoscopic incisions, unless severe pleural adhesion is encountered.
Telaranta endoscopically reconstructed bilateral thoracic sympathetic nerves with interpositional sural nerve grafts on one patient suffering from CH following ETS. Patient’s symptoms and QoL improved by 2.5 years postop, substantiated by normalized sweating pattern measured with vapometer (15). Hamm et al. performed reversal surgery on 19 patients by transposing distally-based intercostal nerve to thoracic sympathetic nerve defects, bilaterally. In all cases, the fibrin sealant was applied onto nerve coaptation anteriorly via endoscopic technique except in one patient who required minithoracotomy owing to dense pleural adhesion. Of the 19 patients, nine reported mild to definite resolution of CH symptoms by an average of 1.83 years (16).
Miura, whilst resecting a mediastinal tumor via posterolateral thoracotomy, successfully reconstructed a 3 cm defect in the thoracic sympathetic chain by transferring the intercostal nerve and performing neurorrhaphy with 6/0 nylon and fibrin glue reinforcement (19).
Other studies have also shown reparability of sympathetic chains using nerve grafts. Kim et al. primarily reconstructed resected cavernous nerves bilaterally with interpositional sural nerve grafts during radical retropubic prostatectomy (20). Postoperative results observed at 18 months were promising. Animal experimental study performed by Hyochi et al. (21) demonstrated comparable restoration of sympathetic pathways via the hypogastric nerve by using both autonomic nerve graft (colonic nerve) and somatic nerve graft (genitofemoral).
The idea of using vascular conduits for nerve repair dates back to 1891 (22). The use of autogenous venous nerve conduit (AVNC) was not recognized as an alternative to nerve grafting until 1982 when Chiu et al. (23) attested that it facilitated regeneration of the proximal nerve fascicles in an orderly fashion within the lumen of the vein which ultimately reached the distal stumps within two months after repair. Subsequently, nerve conduction studies confirmed restoration of conduction through vein-grafted nerves although somewhat slower than baseline. However, this was comparable to nerve-grafted defects (24). Although initial experiments were carried out on animals, numerous human studies followed, illustrating recovery of sensation after bridging digital nerve defects with AVNC, in both immediate and delayed repairs (25-29).
Harvesting of superficial vein grafts is technically uncomplicated with unlimited supply, leaving behind only a small linear scar. Furthermore, the lumen of a vein allows diffusion of the neurotropic factors within, creating an ideal environment for nerve regeneration and prevent scar tissue from invading the lumen (30,31). As a result, veins may be preferred over synthetic conduits such as silicone and Maxon tubes which can cause foreign body reactions and axonal compression, leading to scar formation (32).
There are few limiting factors to successful nerve reconstruction using AVNC. Both animal and human studies demonstrated poor clinical and histological nerve regeneration when AVNC was used to bridge large-diameter, mixed nerves, and those with gaps of more than 3 cm (25-27,33). This is theoretically attributed to dilutional effect of neurotropic factors within longer AVNC, which result in stunted axonal growth (34). When nerve defects are longer than 3 cm, interpositional nerve grafts were shown to be superior to AVNCs (31).
The assessment tools used for long-term outcomes following reversal surgery in our patients were DLQI and the QoL questionnaires. The DLQI, the first dermatology-specific health-related QoL questionnaire, was developed by Finlay and Khan in 1994 (35). It is a well-validated outcome measure for numerous dermatological conditions including primary hyperhidrosis (36,37) and CH (38).
QoL questionnaire, which was introduced by Amir et al. (18) and adapted by de Campos et al., has been frequently employed in evaluating post-ETS cohorts (39-41).
In our two cases, the overall improvement in DLQI and QoL scores were evident, although in varying degrees.
Conclusions
The limited experience in the use of the Melbourne technique demonstrates its promise as a safe treatment and effective option for CH with minimal donor site morbidity. Although it is an uncommon condition, more patients who underwent this technique of reconstruction will need to be recruited and reviewed in order to shed more light on its success.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg 2007;33:908-23. [PubMed]
- Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 2004;51:241-8. [PubMed]
- Stolman LP. Treatment of hyperhidrosis. Dermatol Clin 1998;16:863-9. [PubMed]
- Sato K, Kato T. Chemoembolization using microencapsulated anticancer drugs. Nihon Rinsho 1989;47:1379-83. [PubMed]
- Kux EF. Endoscopic resection of the autonomic nervous system of the thoracic cavity. Rev Esp Tuberc 1951;20:19-24. [PubMed]
- Gjerris F, Olesen HP. Palmar hyperhidrosis. Long-term results following high thoracic sympathectomy. Acta Neurol Scand 1975;51:167-72. [PubMed]
- Kopelman D, Hashmonai M. The correlation between the method of sympathetic ablation for palmar hyperhidrosis and the occurrence of compensatory hyperhidrosis: a review. World J Surg 2008;32:2343-56. [PubMed]
- Lai YT, Yang LH, Chio CC, et al. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery 1997;41:110-3; discussion 113-5. [PubMed]
- Chou SH, Kao EL, Lin CC, et al. The importance of classification in sympathetic surgery and a proposed mechanism for compensatory hyperhidrosis: experience with 464 cases. Surg Endosc 2006;20:1749-53. [PubMed]
- Dewey TM, Herbert MA, Hill SL, et al. One-year follow-up after thoracoscopic sympathectomy for hyperhidrosis: outcomes and consequences. Ann Thorac Surg 2006;81:1227-32; discussion 1232-3. [PubMed]
- Doolabh N, Horswell S, Williams M, et al. Thoracoscopic sympathectomy for hyperhidrosis: indications and results. Ann Thorac Surg 2004;77:410-4; discussion 414. [PubMed]
- Lin TS, Kuo SJ, Chou MC. Uniportal endoscopic thoracic sympathectomy for treatment of palmar and axillary hyperhidrosis: analysis of 2000 cases. Neurosurgery 2002;51:S84-7. [PubMed]
- Moya J, Ramos R, Morera R, et al. Results of high bilateral endoscopic thoracic sympathectomy and sympatholysis in the treatment of primary hyperhidrosis: a study of 1016 procedures. Arch Bronconeumol 2006;42:230-4. [PubMed]
- Shelley WB, Florence R. Compensatory Hyperhidrosis after Sympathectomy. N Engl J Med 1960;263:1056-8.
- Telaranta T. Reversal surgery for reducing the side effects of ETS. A case report. Ann Chir Gynaecol 2001;90:175-6. [PubMed]
- Haam SJ, Park SY, Paik HC, et al. Sympathetic nerve reconstruction for compensatory hyperhidrosis after sympathetic surgery for primary hyperhidrosis. J Korean Med Sci 2010;25:597-601. [PubMed]
- Hyperhidrosis Disease Severity Scale. International Hyperhidrosis Society, 2007. Available online: http://www.sweathelp.org/pdf/HDSS.pdf
- Amir M, Arish A, Weinstein Y, et al. Impairment in quality of life among patients seeking surgery for hyperhidrosis (excessive sweating): preliminary results. Isr J Psychiatry Relat Sci 2000;37:25-31. [PubMed]
- Miura J, Doita M, Miyata K, et al. Horner’s syndrome caused by a thoracic dumbbell-shaped schwannoma: sympathetic chain reconstruction after a one-stage removal of the tumor. Spine (Phila Pa 1976) 2003;28:E33-6. [PubMed]
- Kim ED, Scardino PT, Kadmon D, et al. Interposition sural nerve grafting during radical retropubic prostatectomy. Urology 2001;57:211-6. [PubMed]
- Hyochi N, Kihara K, Arai G, et al. Reconstruction of the sympathetic pathway projecting to the prostate, by nerve grafting in the dog. BJU Int 2004;94:147-52. [PubMed]
- Buengner OV. Ueber die Degenerations-und Regenerations-vorgaenge am Nerven nach Verletzungen. Beitr Pathol Anat 1891;10:321.
- Chiu DT, Janecka I, Krizek TJ, et al. Autogenous vein graft as a conduit for nerve regeneration. Surgery 1982;91:226-33. [PubMed]
- Chiu DT, Lovelace RE, Yu LT, et al. Comparative electrophysiologic evaluation of nerve grafts and autogenous vein grafts as nerve conduits: an experimental study. J Reconstr Microsurg 1988;4:303-9, 311-2. [PubMed]
- Walton RL, Brown RE, Matory WE Jr, et al. Autogenous vein graft repair of digital nerve defects in the finger: a retrospective clinical study. Plast Reconstr Surg 1989;84:944-9; discussion 950-2. [PubMed]
- Chiu DT, Strauch B. A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast Reconstr Surg 1990;86:928-34. [PubMed]
- Tang JB, Gu YQ, Song YS. Repair of digital nerve defect with autogenous vein graft during flexor tendon surgery in zone 2. J Hand Surg Br 1993;18:449-53. [PubMed]
- Calcagnotto GN, Braga Silva J. The treatment of digital nerve defects by the technique of vein conduit with nerve segment. A randomized prospective study. Chir Main 2006;25:126-30. [PubMed]
- Lee YH, Shieh SJ. Secondary nerve reconstruction using vein conduit grafts for neglected digital nerve injuries. Microsurgery 2008;28:436-40. [PubMed]
- Raivich G, Kreutzberg GW. Expression of growth factor receptors in injured nervous tissue. I. Axotomy leads to a shift in the cellular distribution of specific beta-nerve growth factor binding in the injured and regenerating PNS. J Neurocytol 1987;16:689-700. [PubMed]
- Raivich G, Zimmermann A, Sutter A. Nerve growth factor (NGF) receptor expression in chicken cranial development. J Comp Neurol 1987;256:229-45. [PubMed]
- Meek MF, Coert JH. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg 2008;60:110-6. [PubMed]
- Moore AM, Kasukurthi R, Magill CK, et al. Limitations of conduits in peripheral nerve repairs. Hand (N Y) 2009;4:180-6. [PubMed]
- Taniuchi M, Clark HB, Schweitzer JB, et al. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci 1988;8:664-81. [PubMed]
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6. [PubMed]
- Swartling C, Naver H, Lindberg M. Botulinum A toxin improves life quality in severe primary focal hyperhidrosis. Eur J Neurol 2001;8:247-52. [PubMed]
- Campanati A, Penna L, Guzzo T, et al. Quality-of-life assessment in patients with hyperhidrosis before and after treatment with botulinum toxin: results of an open-label study. Clin Ther 2003;25:298-308. [PubMed]
- Kim WO, Kil HK, Yoon KB, et al. Botulinum toxin: a treatment for compensatory hyperhidrosis in the trunk. Dermatol Surg 2009;35:833-8; discussion 838. [PubMed]
- Ishy A, de Campos JR, Wolosker N, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg 2011;12:545-8. [PubMed]
- Yazbek G, Wolosker N, Kauffman P, et al. Twenty months of evolution following sympathectomy on patients with palmar hyperhidrosis: sympathectomy at the T3 level is better than at the T2 level. Clinics (Sao Paulo) 2009;64:743-9. [PubMed]
- de Campos JR, Kauffman P, Werebe Ede C, et al. Quality of life, before and after thoracic sympathectomy: report on 378 operated patients. Ann Thorac Surg 2003;76:886-91. [PubMed]


