
Role of Kindlin-2 in cancer progression and metastasis
Introduction
Kindlins are 4.1-ezrin-ridixin-moesin (FERM) domain containing proteins, which are common proteins that are involved in the localization of proteins to the plasma membrane (1). The FERM domain stands for F: 4.1 protein; E; ezrin; R: radixin; and M: moesin. The FERM domain can be located in several scaffolding proteins at the interface between the plasma membrane and the cytoskeleton (2). There are three members in the Kindlins family: Kindlin-1, Kindlin-2 (K2), and Kindlin-3. Kindlin-1 is known as FERMT1 that maps to chromosome 20p12.3, K2 is known as FERMT2 and is located to chromosome 14q22.1, and Kindlin-3, also known as FERMT3 and maps to chromosome 11q13.1. Each of the 3 mammalian Kindlins contains four FERM domains (F0–F3). Kindlins can be distinguished from other FERM domain-containing proteins by the presence of a PH domain that splits the F2 domain. This PH domain helps target Kindlins to membranes (3,4), which, together with other lipid binding subdomains (5), facilitates the interactions and activation of integrins (6). The 3 Kindlin family members share more than 50% in sequence identity (5,7,8). However, K2 is more likely to have retained the original features of the Kindlins’ family while kindlin-1 and -3 originated from reproduction of the K2 gene. The Kindlins can, however, be distinguished by the presence of variable regions that are interspersed within the conserved regions (9). The length and extent of these variable regions are most diverse in K2 and may account for the differences in the functions of K2, compared to Kindlin-1 and Kindlin-3. In recent years, publications on Kindlins have exploded from a mere dozen publications in 2008 to more than 380 (December 2019), with studies on K2 alone account for more than half. Reviews emphasizing K2 have been published in top-tier journals (7,9-13). Interest in K2 stemmed from in vitro studies which demonstrated that K2 can coordinate with Talin head to stimulate activation of integrins in cells (14,15) and from the phenotype of K2-deficient mice; homozygous deletion of K2 is embryonically lethal. Furthermore, recent studies have established K2 as a major driver of progression and metastasis of tumors by controlling several hallmarks of cancer (16-18). Collectively, these studies establish the foundation and relevance of K2 to human biology, both in physiological and pathological settings.
K2 and the cancer progression-metastasis cascade
One sole malefactor for most cancer-related deaths is metastasis. This appears to be the ultimate challenge in the battle against cancer (19). K2 is the most broadly distributed member of the kindlin family. K2 was found to regulate tumor progression and metastasis by controlling numerous signaling pathways that are required for survival, proliferation, migration, invasion and metastasis of cancer cells (16-18). Moreover, K2 has been unquestionably associated with almost every hallmark of cancer (17,18). K2 is overexpressed in several cancers of multiple origins, including the prostate (20,21), breast (22), lung (23,24), pancreas (25,26), esophagus (27-29), liver (30), brain (31), the gastric system (32,33), and the bladder (34). K2 expression levels are positively correlated with the aggressiveness of breast cancer tumors (35). In fact, K2 was associated with triple-negative breast cancer (TNBC) tumors, which belong to a subtype of breast cancer tumors that are especially devastating due to their highly metastatic behavior, their propensity to recur rapidly, and to their low response to standard-of-care chemotherapies. K2 was also considered as one of the risk factors for lymph node metastasis in breast cancer because of higher expression levels in lymph nodes of breast cancer patients (35). Blocking K2 interaction with DNA methyltransferase1 (DNMT1) suppressed breast cancer metastasis (36). In gastric cancer, K2 expression showed positive correlation with TNM stage, invasion depth, and lymph node metastasis, all of which are associated with poor overall survival (37). Overexpression of K2 is also associated with metastasis, high tumor grade, recurrence, and lack of response to chemotherapy in patients with osteosarcoma (37). High expression of K2 was significantly associated with late-stage metastasis in patients with hepatocellular carcinoma (HCC). K2 promotes HCC cell migration and invasion through up-regulation of Wnt-β-catenin signaling, which enhances epithelial-mesenchymal transition (38). Increased expression of K2 was reported in aggressive malignant mesothelioma tumors and K2 was found to enhance cell migration and invasion of mesothelioma cancer cells (39). Other reports also showed that high expression of K2 was involved in promoting tumor invasion-metastasis and was associated with poor disease outcome in patients with clear cell renal cell tumors (40). Zhan et al. found that K2 plays a key part in promoting the progression and invasion of pancreatic ductal adenocarcinoma (26). However, to date, only a single study has suggested that K2 may have a tumor suppressor activity. Ren et al. reported that while K2 was highly expressed in normal tissues, its expression levels were low in tumor tissues of serous epithelial ovarian cancer (41). Therefore, based on this body of literature, it is now clear that K2 can be classified as a major driver of cancer progression and metastasis.
K2 and the epithelial-mesenchymal transition program
Epithelial-mesenchymal transition (EMT) is the process by which epithelial cells trans-differentiate into more motile mesenchymal phenotype (42). EMT also plays an essential role in the acquisition of the metastatic phenotype of cancer tumors (42). EMT is the biological process whereby cancer cells gain an invasive phenotype by going through a morphologic change from a differentiated to a more dedifferentiated phenotype (43), which enables stationary epithelial cells to gain the ability to migrate from the primary tumor and invade organs and tissues (44). In addition, it is a critical process during embryonic development and wound healing (42). The EMT programs are now recognized as major drivers of metastasis in several cancers, doing so through remodeling the actin cytoskeleton from apical–basolateral phenotype that is characteristic of epithelial cells, to a more mesenchymal phenotype through synthesis of actin stress fibers (45). Consequently, EMT programs incur immotile epithelial cells to attain migratory-invasive phenotypes, as well as displaying a cancer stemness-like phenotype and chemo-resistant behaviors. K2 regulates the invasion-metastasis cascade by modulating several signaling pathways, the most significant of which is EMT. A direct link between K2 and the TGF-β/EMT axis is illustrated in study by Yu et al., where K2 was found to control the activity of micro-RNA-200 (miR-200b), a well-established master regulator of EMT (46). K2 was found to form a complex with DNMT3A that occupy the promoter of miR-200b, thereby inducing CpG island hypermethylation, which results in the inhibition of miR-200b expression. This K2-mediated epigenetic repression of miR-200, and therefore activation of EMT, was found to promote breast cancer invasion. Members of the miR-200 family also regulate EMT by controlling the expression of EMT principal transcription factors ZEB1 and ZEB2 (47). In fact, a double-negative feedback loop between microRNA miR-200 family and ZEB1/2 was described to tightly regulate expression of both miR200 and ZEB1/2 during EMT (48). Accordingly, K2 was found to activate ZEB2, which in turn inhibits expression of miR-200b, and therefore, activates the EMT-mediated stimulation of migration and invasion of oral squamous cell carcinoma (49).
K2, E-cadherin and EMT
A common manifestation of EMT is the down-regulation of E-cadherin which leads to the disruption of adherens junctions that “stick” cells together in a very fashionable structure (44). This loss of E-cadherin allows cancer cells to acquire a mesenchymal phenotype, that is associated with increased cell migration and invasion, which is also concomitant to the “Cadherins’ Switch”, where increased expression of other types of Cadherins, such as N-Cadherin, R-Cadherin, P-Cadherin, takes place (50). K2 was also described to associate with DNA methyltransferase 1 (DNMT1) and enhance its occupancy of the E-cadherin promoter, resulting in the hypermethylation of CpG islands and suppression of E-cadherin expression, and therefore activation of EMT (36). Also, K2 induces EMT through up-regulation of estrogen receptor α (ERα). ERα is then recruited to the E-cadherin promoter and suppresses its transcription (41). Together, the literature supports the notion that K2 regulates EMT through its modulation of E-Cadherin expression.
K2, TGF-β signaling and EMT
TGF-β is a multifunctional cytokine that has been established to regulate several stages of development (51). Furthermore, TGF-β suppresses the transformation and tumor formation of several cancers of epithelial origin, and therefore, acts as a tumor suppressor (51). With the evolution and progression of tumors, TGF-β generally loses its tumor-suppressor behaviors and switches to a tumor-promoting function (52-54), by enhancing cancer proliferation, migration-invasion, and metastasis through the activation of EMT programs (52-54). The switch from tumor suppressor to tumor promoter is referred to as the TGF-β paradox (55). Accordingly, countless studies have established TGF-β as a key regulator of EMT in different normal and tumor cells and tissues (44). However, the accurate queue of events that trigger the switch of TGF-β function from tumor suppressor to oncogene during tumorigenesis remains largely unknown. Moreover, the molecular mechanisms by which TGF-β induces EMT programs is still undisclosed. This gap of knowledge is further complicated by the capability of TGF-β to switch from a canonical to a non-canonical signaling; i.e., from SMAD2/3/4-dependent to SMAD independent, both of which amalgamate in promoting EMT induced by TGF-β (44,50,52).
In cancer, increased expression and activation of TGF-β1 was found to promote epithelial plasticity that ultimately may progress to EMT programs, an essential transition for cancer cell during migration-invasion and dissemination (52). In response to TGF-β, complexes of SMAD activate the expression and increase the capability of transcription factors in EMT programs (56). Kindlins were found to promote TGF-β signaling through up-regulation of Smad2 and Smad3 phosphorylation. High expression of K2 increased TGF-β-stimulated activation of Smad3, while knockdown of K2 inhibited the interaction between Smad3 and TGF-β receptor I (TβRI) and, therefore, suppressed TGF-β-mediated activation of Smad3 (57,58). Kindlin-1 was also documented as an important regulator of TGF-β signaling. Kindlin-1 was found to be required for the interaction between TβRI and Smad3. This interaction is necessary for the TGF-β-mediated phosphorylation of SMAD3 and its subsequent nuclear translocation, which ultimately results in activation of the EMT program (59). K2 has also been associated with TGF-β-mediated activation of EMT in cancer. In fact, a positive feedback loop between TGF-β and K2 has been described to play a major function in triggering the progression and metastasis of TNBC, through the promotion of EMT reactions (16). A recent study found a pivotal relationship between TβRI and K2, which was established to be prerequisite for the stabilization of TβRI and activation of migration—invasion of breast cancer in vitro, and metastasis in vivo (16). In esophageal squamous cell carcinoma, dysregulation of K2 expression was found to play an important role in EMT through activation of TGF-β signaling (29). The positive feed-back loop between K2 and TGF-β was also described in pancreatic ductal adenocarcinoma (PDAC) cells, where K2 was found to upregulate TβRI resulting in the activation of TGF-β signaling, which in turn, enhances K2 expression (60). In breast cancer, it has been discovered that K2 is required for the recruitment of macrophages to the primary tumor and their polarization to the anti-inflammatory type II macrophages, through the activation of the TGF-β/CSF-1/EGF paracrine signaling pathway. This vicious oncogenic paracrine loop has been described to feed into the activation of tumor growth, progression and metastasis of breast cancer tumors (16). A report found that K2 to induce cancer-associated fibroblasts (CAFs), which triggers the invasiveness of bladder cancer cells by activating the EMT programs downstream of TGF-β (61). In addition, K2 has been established to regulate TGF-β signaling and SOX9 expression which is a cancer stemness transcription factor to control chondrogenesis (62). These findings demonstrate that K2 controls the metastatic progression of cancer tumors through the regulation of TGF-β signaling. Other studies also showed that K2 causes suppression of the integrin β1-AKT signaling pathway by targeting K2 to ZEB1/2, which is an important hallmark of EMT axis (27).
K2 and cancer stem cells (CSCs)
CSCs are a subpopulation of cancer cells with stemness properties that contribute to tumor initiation and progression, therapy resistance and metastatic dissemination. In normal physiological conditions, increased expression levels of kindlins in embryonic stem cells, were found to play a key role in the determination of early stage embryonic (63). Rognoni et al. showed that Kindlin-1 regulated homeostasis of cutaneous epithelial stem cells through activating αvβ6 integrin-mediated TGF-β release by suppressing Wnt-β-catenin pathway through an integrin-independent regulation of expression of Wnt ligand in the skin cells (64). K2 triggered the pluripotency mesenchymal stromal-derived cells (iPSC-MSCs), leading to increase proliferation, survival, migration-invasion, stemness and decreased apoptosis (65). Furthermore, K2 is an important factor in fate-decision of MSC through control of YAP1/TAZ (66), while Kindlin-3-mediated integrin activation can regulate the fate of hematopoietic stem cells (HPSC) in the bone marrow, while preserving the activated state of HPSCs and hematopoietic progenitor cells (67). Therefore, Kindlins are involved in the regulation of cell stemness. K2 also regulates stemness in cancer through Wnt/Beta-catenin and Hedgehog pathway, which are the hallmarks of cancer stem cells (42,43).
K2 and Wnt signaling
Wnt signaling has several identified components and processes. These components and processes include the process of destruction of the β-catenin complex, Wnt secretory machinery, and Wnt co-receptors and nuclear co-factors, all of which are required for the regulation of stemness of cancer cells (42,43). K2 forms a transcriptional complex with TCF4 and β-catenin which increases Wnt signaling during breast cancer progression (68). K2 also activates Wnt/β-catenin signaling and enhances expression of non-phosphorylated β-catenin, as well as Axin2 and MMP7 which were two targets of the Wnt/β-catenin signaling pathway (38). K2 controls myogenic regulatory factors myogenin through canonical Wnt/β-catenin signaling. K2 forms a three-way complex with activated β-catenin and TCF4, and co-occupy the promoter of myogenin to increase its expression (69). Li et al. overexpressed K2 in the mammary gland of mice and found K2 promoted tumor formation and growth through activation of the Wnt/β-catenin signaling pathway (70). Together, these results indicate that K2 promotes initiation and maintains stemness of cancers through activation of Wnt/β-catenin signaling.
Other Kindlin family members were also found to be involved in the Wnt signaling. Kindlin-1 was reported to control keratinocyte adhesion through β1 integrin, and to activate proliferation and differentiation of cutaneous epithelial stem cells through facilitating αvβ6 integrin-mediated activation of TGF-β, and through suppressing Wnt-β-catenin signaling pathway in an integrin-independent regulation of Wnt ligand expression (64). On the other hand, Kindlin-3 was reported to promote resistance of glioma stem cells to temozolomide through activation of Wnt signaling (71).
K2 and Hedgehog (Hh) signaling
Uncontrolled Hh pathway activation is linked to the initiation and development of several types of cancers (72). K2 was reported to regulate GLI1, a known hedgehog signaling pathway effector. K2 can promote GLI1 expression through inactivation of glycogen syntheses kinase 3β (73). In turn, K2 may be down-regulated though GLI1 occupation on the K2 promoter at the transcriptional level (73). This K2-GLI1 crosstalk enables the sensitization of cancer cells to cyclopamine-induced cell death of prostate cancer cells (73).
Mechanisms of regulation of K2
miR200, K2 and EMT
The miR-200 family, which is composed of 5 members (miR-200a, -200b, -200c, -141, -429), is a family of miRNAs that has been demonstrated to play key roles in EMT in cancer (47,74-76). Sossey-Alaoui group found that regulation of EMT by K2 was mediated through miR-200b. miR-200b specifically targets and controls expression of K2 via a conserved seed sequence in the 3'UTR in the human and mouse K2 (FERM2) genes. Furthermore, miR-200b expression levels negatively associate with that of K2 and with the metastasis-aggressiveness of breast cancer cell lines. The miR-200b-mediated inhibition of K2 manifested in the regulation of the EMT phenotype (77). Zhang lab, on the other hand, found that loss of miR-200b downstream of K2/integrin β1/AKT signaling, enhances invasion of esophageal squamous cell carcinoma cells (ESCC). Targeted-inhibition of K2 lifted the suppression on miR-200b, which in turn suppressed metastasis-invasion of esophageal squamous cell carcinoma (ESCC) cells (27).
MiR138-K2 and resistance to therapy
MiR138 was linked to drug-resistance in various cancers, including myeloma, renal cell carcinoma, breast cancer, non-small cell lung cancer by regulation of different signaling, such as EMT and PI3K-AKT (78-80). Sossey-Alaoui lab discovered that miR-138 specifically targeted K2 and suppressed its expression, accordingly, modulating a miR-138/K2/β1-integrin signaling axis in metastatic castration-resistant prostate cancer that is crucial for the sensitization to chemotherapeutics (21).
Src tyrosine Kinase, K2 and integrins
Phosphorylation of amino acid residues within functional domains of proteins is an established active regulatory mechanism of these proteins. As such, K2 is targeted by Src non-receptor tyrosine kinase to specifically phosphorylate K2 at tyrosine residue Y193 and this Src-K2 interaction was found to control integrin outside-in signaling and to regulate cell proliferation and migration (81). In turn, K2 phosphorylation at Y193 was determined to be essential for the maintenance of Src kinase activity (71).
Cytoplasmic vs. nuclear function of K2
Kindlin 2, as an integrin-associated protein (strictly cytoplasmic), was recently described to have functions in both the cytoplasm/membrane as well as in the nucleus. Loss of K2 suppresses activation of RhoA and inhibits phosphorylation of myosin light-chain, formation of stress fibers, and focal adhesion assembly, leading to increased phosphorylation of Ser127, nuclear exclusion, and ubiquitin ligase atrophin-1 interacting protein 4-mediated degradation of YAP1/TAZ (66). K2 was also shown to activate nuclear factor (NF)-κB-dependent pathway and leading to enhance the matrix metalloproteinase-9 and-2 expression to promote invasiveness of prostate cancer (20). The most studied function of K2 focused on their role in activation of integrin. Activation of integrin relays on inside-out signaling, as a result of the cooperation and binding of talin and kindlin to the cytoplasmic domain of integrins. Loss of K2 affects the interaction of β1 integrin with α-actinin-2 and fractures the Z-disk structure (82). K-2 extreme C terminus is vital for integrin co-activation and emphasizes the importance of an atypical structure of the FERM-2 domain in controlling activation of integrin (83). In the nucleus, nuclear translocation of β-catenin and K2 was triggered during myogenic differentiation. Sossey-Alaoui lab found that K2 regulated senescence in part by K-2 interaction with p53, where it modulated the p53-responsive genes’ expression, during the induction of cellular senescence in breast cancer (18). Interactional relationship between K2 and p53 at the promoter level was detected to be crucial for the modulated SerpinB2 and p21 expression during cancer cell senescence (18).
Conclusions and outlook
During the last few years, K2 has gained tremendous interest, thanks to its strong relationship with metastasis and cancer cell stemness in several types of cancers. K2 promotes tumor initiation, development, resistance to standard of care therapies, and metastatic dissemination. Mechanistically, K2 plays a key role in the regulation of EMT, TGF-β, Wnt/β-Catenin, Hedgehog signaling (Figure 1). Overall, K2 has been documented to be associated with almost every hallmark of cancer (84). For these reasons listed above, K2 is considered a potential target for therapeutic targeting aimed at overcoming cancer progression and metastasis.
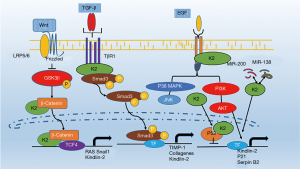
Moreover, K2 may be a potential target in cancer immunotherapy. The recent successes of some clinical trials using immune checkpoint therapy, have been credited to the use of blocking antibodies to target cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1), and through chimeric antigen receptor (CAR) T cells. These are few positive efforts aimed to tip the scales contributing to the immune system in the elimination of various cancer cells (85). We hypothesize that K2, by regulating TGF-β-PD-L1 feedback loop, may also play a crucial role in the regulation of cancer immunotherapy. It is well known that K2 regulates tumor metastasis and cancer stemness through TGF-β signaling. TGFβ, on the other hand, shapes the tumor microenvironment to suppress anti-tumor immunity by inhibiting T-cell infiltration (86). Since TGF-β silencing was found to restore the efficacy of anti-PD-1/anti-CTLA-4 treatment (87), as the adaptor of TGF-β, K2 may present to be an important player in cancer immunotherapy.
Acknowledgments
Funding: This work was supported by funding from the National Institute of Health (NIH) [Grant No. 7R01CA226921]; and by startup funds from the MetroHealth System to KSA.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Khalid Sossey-Alaoui) for the Series “Cancer Metastasis: Molecular signaling and therapeutic options” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.03.64). The series “Cancer Metastasis: Molecular signaling and therapeutic options” was commissioned by the editorial office without any funding or sponsorship. KSA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Translational Medicine from Sep 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chishti AH, Kim AC, Marfatia SM, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 1998;23:281. [Crossref] [PubMed]
- Pearson MA, Reczek D, Bretscher A, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000;101:259-70. [Crossref] [PubMed]
- Liu J, Fukuda K, Xu Z, et al. Structural basis of phosphoinositide binding to kindlin-2 protein pleckstrin homology domain in regulating integrin activation. J Biol Chem 2011;286:43334-42. [Crossref] [PubMed]
- Liu Y, Zhu Y, Ye S, et al. Crystal structure of kindlin-2 PH domain reveals a conformational transition for its membrane anchoring and regulation of integrin activation. Protein Cell. 2012;3:434-40. [Crossref] [PubMed]
- Perera HD, Ma Y-Q, Yang J, et al. Membrane binding of the N-terminal ubiquitin-like domain of kindlin-2 is crucial for its regulation of integrin activation. Structure 2011;19:1664-71. [Crossref] [PubMed]
- Metcalf DG, Moore DT, Wu Y, et al. NMR analysis of the αIIbβ3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc Natl Acad Sci 2010;107:22481-6. [Crossref] [PubMed]
- Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell–matrix adhesion. EMBO Rep 2008;9:1203-8. [Crossref] [PubMed]
- Moser M, Nieswandt B, Ussar S, et al. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med 2008;14:325-30. [Crossref] [PubMed]
- Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood 2010;115:4011-7. [Crossref] [PubMed]
- Brahme NN, Calderwood DA. Cell adhesion: a FERM grasp of the tail sorts out integrins. Curr Biol 2012;22:R692-4. [Crossref] [PubMed]
- Meves A, Stremmel C, Gottschalk K, et al. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol 2009;19:504-13. [Crossref] [PubMed]
- Moser M, Legate KR, Zent R, et al. The tail of integrins, talin, and kindlins. Science 2009;324:895-9. [Crossref] [PubMed]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 2010;11:288-300. [Crossref] [PubMed]
- Ma Y-Q, Qin J, Wu C, et al. Kindlin-2 (Mig-2): a co-activator of β3 integrins. J Cell Biol 2008;181:439-46. [Crossref] [PubMed]
- Ye F, Snider AK, Ginsberg MH. Talin and kindlin: the one-two punch in integrin activation. Front Med 2014;8:6-16. [Crossref] [PubMed]
- Sossey-Alaoui K, Pluskota E, Bialkowska K, et al. Kindlin-2 Regulates the Growth of Breast Cancer Tumors by Activating CSF-1–Mediated Macrophage Infiltration. Cancer Res 2017;77:5129-41. [Crossref] [PubMed]
- Sossey-Alaoui K, Pluskota E, Davuluri G, et al. Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis. FASEB J 2014;28:2260-71. [Crossref] [PubMed]
- Sossey-Alaoui K, Pluskota E, Szpak D, et al. The Kindlin2-p53-SerpinB2 signaling axis is required for cellular senescence in breast cancer. Cell Death Dis 2019;10:539. [Crossref] [PubMed]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med 2013;19:1450. [Crossref] [PubMed]
- Yang JR, Pan T, Yang H, et al. Kindlin-2 promotes invasiveness of prostate cancer cells via NF-κB-dependent upregulation of matrix metalloproteinases. Gene 2016;576:571-6. [Crossref] [PubMed]
- Sossey-Alaoui K, Plow EF. miR-138–Mediated Regulation of KINDLIN-2 Expression Modulates Sensitivity to Chemotherapeutics. Mol Cancer Res 2016;14:228-38. [Crossref] [PubMed]
- Zhao T, Guan L, Yu Y, et al. Kindlin-2 promotes genome instability in breast cancer cells. Cancer Lett 2013;330:208-16. [Crossref] [PubMed]
- Zhan J, Zhu X, Guo Y, et al. Opposite role of Kindlin-1 and Kindlin-2 in lung cancers. PLoS One 2012;7:e50313. [Crossref] [PubMed]
- Guo L, Cui C, Zhang K, et al. Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nat Commun 2019;10:845. [Crossref] [PubMed]
- Mahawithitwong P, Ohuchida K, Ikenaga N, et al. Kindlin-2 expression in peritumoral stroma is associated with poor prognosis in pancreatic ductal adenocarcinoma. Pancreas 2013;42:663-9. [Crossref] [PubMed]
- Zhan J, Song J, Wang P, et al. Kindlin-2 induced by TGF-β signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Cancer Lett 2015;361:75-85. [Crossref] [PubMed]
- Zhang HF, Alshareef A, Wu C, et al. Loss of miR-200b promotes invasion via activating the Kindlin-2/integrin β1/AKT pathway in esophageal squamous cell carcinoma: An E-cadherin-independent mechanism. Oncotarget 2015;6:28949. [Crossref] [PubMed]
- Cao HH, Zhang SY, Shen JH, et al. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget 2015;6:5435. [Crossref] [PubMed]
- Zhang H-F, Zhang K, Liao L-D, et al. miR-200b suppresses invasiveness and modulates the cytoskeletal and adhesive machinery in esophageal squamous cell carcinoma cells via targeting Kindlin-2. Carcinogenesis 2014;35:292-301. [Crossref] [PubMed]
- Ge YS, Liu D, Jia WD, et al. Kindlin-2: a novel prognostic biomarker for patients with hepatocellular carcinoma. Pathol-Res Pract 2015;211:198-202. [Crossref] [PubMed]
- Ou YW, Zhao Z, Wu C, et al. Mig-2 attenuates cisplatin-induced apoptosis of human glioma cells in vitro through AKT/JNK and AKT/p38 signaling pathways. Acta Pharmacol Sin 2014;35:1199-206. [Crossref] [PubMed]
- Shen Z, Ye Y, Dong L, et al. Kindlin-2: a novel adhesion protein related to tumor invasion, lymph node metastasis, and patient outcome in gastric cancer. Am J Surg 2012;203:222-9. [Crossref] [PubMed]
- Shen Z, Ye Y, Kauttu T, et al. Novel focal adhesion protein kindlin‐2 promotes the invasion of gastric cancer cells through phosphorylation of integrin β1 and β3. J Surg Oncol. 2013;108:106-12. [Crossref] [PubMed]
- Talaat S, Somji S, Toni C, et al. Kindlin-2 expression in arsenite-and cadmium-transformed bladder cancer cell lines and in archival specimens of human bladder cancer. Urology 2011;77:1507.e1-7. [Crossref] [PubMed]
- Xue X, Li J, Wan W, et al. Kindlin-2 could influence breast nodule elasticity and improve lymph node metastasis in invasive breast cancer. Sci Rep 2017;7:6753. [Crossref] [PubMed]
- Wang P, Chu W, Zhang X, et al. Kindlin-2 interacts with and stabilizes DNMT1 to promote breast cancer development. Int J Biochem Cell Biol 2018;105:41-51. [Crossref] [PubMed]
- Ning K, Zhang H, Wang Z, et al. Prognostic implications of Kindlin proteins in human osteosarcoma. Onco Targets Ther 2017;10:657. [Crossref] [PubMed]
- Lin J, Lin W, Ye Y, et al. Kindlin-2 promotes hepatocellular carcinoma invasion and metastasis by increasing Wnt/β-catenin signaling. J Exp Clin Cancer Res 2017;36:134. [Crossref] [PubMed]
- An Z, Dobra K, Lock JG, et al. Kindlin‐2 is expressed in malignant mesothelioma and is required for tumor cell adhesion and migration. Int J Cancer 2010;127:1999-2008. [Crossref] [PubMed]
- Yan M, Zhang L, Wu Y, et al. Increased expression of kindlin‐2 is correlated with hematogenous metastasis and poor prognosis in patients with clear cell renal cell carcinoma. FEBS Open Bio 2016;6:660-5. [Crossref] [PubMed]
- Ren C, Du J, Xi C, et al. Kindlin-2 inhibits serous epithelial ovarian cancer peritoneal dissemination and predicts patient outcomes. Biochem Biophys Res Commun 2014;446:187-94. [Crossref] [PubMed]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial‐mesenchymal transition. Bioessays 2001;23:912-23. [Crossref] [PubMed]
- Berx G, Raspé E, Christofori G, et al. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis 2007;24:587-97. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178. [Crossref] [PubMed]
- Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-β in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia 2010;15:169-90. [Crossref] [PubMed]
- Yu Y, Wu J, Guan L, et al. Kindlin 2 promotes breast cancer invasion via epigenetic silencing of the microRNA200 gene family. Int J Cancer 2013;133:1368-79. [Crossref] [PubMed]
- Title AC, Hong SJ, Pires ND, et al. Genetic dissection of the miR-200–Zeb1 axis reveals its importance in tumor differentiation and invasion. Nat Commun 2018;9:4671. [Crossref] [PubMed]
- Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008;68:7846-54. [Crossref] [PubMed]
- Ren W, Gao L, Qiang C, et al. Kindlin-2-mediated upregulation of ZEB2 facilitates migration and invasion of oral squamous cell carcinoma in a miR-200b-dependent manner. Am J Transl Res 2018;10:2529. [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [Crossref] [PubMed]
- Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res 2009;19:156-72. [Crossref] [PubMed]
- Imamura T, Hikita A, Inoue Y. The roles of TGF-β signaling in carcinogenesis and breast cancer metastasis. Breast Cancer 2012;19:118-24. [Crossref] [PubMed]
- Lamouille S, Connolly E, Smyth JW, et al. TGF-β-induced activation of mTOR complex 2 drives epithelial–mesenchymal transition and cell invasion. J Cell Sci 2012;125:1259-73. [Crossref] [PubMed]
- Lamouille S, Derynck R. Cell size and invasion in TGF-β–induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 2007;178:437-51. [Crossref] [PubMed]
- Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 2013;25:76-84. [Crossref] [PubMed]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003;425:577. [Crossref] [PubMed]
- Wei X, Xia Y, Li F, et al. Kindlin-2 mediates activation of TGF-β/Smad signaling and renal fibrosis. J Am Soc Nephrol 2013;24:1387-98. [Crossref] [PubMed]
- Yu J, Hu Y, Gao Y, et al. Kindlin-2 regulates hepatic stellate cells activation and liver fibrogenesis. Cell Death Discov 2018;4:34. [Crossref] [PubMed]
- Kong J, Du J, Wang Y, et al. Focal adhesion molecule Kindlin-1 mediates activation of TGF-β signaling by interacting with TGF-βRI, SARA and Smad3 in colorectal cancer cells. Oncotarget 2016;7:76224. [Crossref] [PubMed]
- Yoshida N, Masamune A, Hamada S, et al. Kindlin-2 in pancreatic stellate cells promotes the progression of pancreatic cancer. Cancer Lett 2017;390:103-14. [Crossref] [PubMed]
- Wu J, Yu C, Cai L, et al. Effects of increased Kindlin-2 expression in bladder cancer stromal fibroblasts. Oncotarget 2017;8:50692. [Crossref] [PubMed]
- Wu C, Jiao H, Lai Y, et al. Kindlin-2 controls TGF-β signalling and Sox9 expression to regulate chondrogenesis. Nat Commun 2015;6:7531. [Crossref] [PubMed]
- Zhan J, Zhang H. Kindlins: roles in development and cancer progression. Int J Biochem Cell Biol 2018;98:93-103. [Crossref] [PubMed]
- Rognoni E, Widmaier M, Jakobson M, et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat Med 2014;20:350. [Crossref] [PubMed]
- Moslem M, Eggenschwiler R, Wichmann C, et al. Kindlin-2 Modulates the Survival, Differentiation, and Migration of Induced Pluripotent Cell-Derived Mesenchymal Stromal Cells. Stem Cells Int 2017;2017:7316354. [Crossref] [PubMed]
- Guo L, Cai T, Chen K, et al. Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. J Cell Biol 2018;217:1431-51. [Crossref] [PubMed]
- Ruppert R, Moser M, Sperandio M, et al. Kindlin-3–mediated integrin adhesion is dispensable for quiescent but essential for activated hematopoietic stem cells. J Exp Med 2015;212:1415-32. [Crossref] [PubMed]
- Yu Y, Wu J, Wang Y, et al. Kindlin 2 forms a transcriptional complex with β‐catenin and TCF4 to enhance Wnt signalling. EMBO Rep 2012;13:750-8. [Crossref] [PubMed]
- Yu Y, Qi L, Wu J, et al. Kindlin 2 regulates myogenic related factor myogenin via a canonical Wnt signaling in myogenic differentiation. PLoS One 2013;8:e63490. [Crossref] [PubMed]
- Li B, Chi X, Song J, et al. Integrin-interacting protein Kindlin-2 induces mammary tumors in transgenic mice. Sci China Life Sci 2019;62:225-34. [Crossref] [PubMed]
- Lu C, Cui C, Liu B, et al. FERMT3 contributes to glioblastoma cell proliferation and chemoresistance to temozolomide through integrin mediated Wnt signaling. Neurosci Lett 2017;657:77-83. [Crossref] [PubMed]
- Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996;384:129-34. [Crossref] [PubMed]
- Gao J, Khan AA, Shimokawa T, et al. A feedback regulation between Kindlin-2 and GLI1 in prostate cancer cells. FEBS Lett 2013;587:631-8. [Crossref] [PubMed]
- Aigner K, Dampier B, Descovich L, et al. The transcription factor ZEB1 (δ EF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007;26:6979-88. [Crossref] [PubMed]
- Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005;24:2375-85. [Crossref] [PubMed]
- Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593-601. [Crossref] [PubMed]
- Sossey-Alaoui K, Pluskota E, Szpak D, et al. The Kindlin-2 regulation of epithelial-to-mesenchymal transition in breast cancer metastasis is mediated through miR-200b. Sci Rep 2018;8:7360. [Crossref] [PubMed]
- Rastgoo N, Pourabdollah M, Abdi J, et al. Dysregulation of EZH2/miR-138 axis contributes to drug resistance in multiple myeloma by downregulating RBPMS. Leukemia 2018;32:2471-82. [Crossref] [PubMed]
- Yun EJ, Zhou J, Lin CJ, et al. The network of DAB2IP-miR-138 in regulating drug resistance of renal cell carcinoma associated with stem-like phenotypes. Oncotarget 2017;8:66975. [Crossref] [PubMed]
- Chen X, Lu P, Wang D, et al. The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene 2016;595:221-6. [Crossref] [PubMed]
- Qu H, Tu Y, Guan J-L, et al. Kindlin-2 tyrosine phosphorylation and interaction with Src serve as a regulatable switch in the integrin outside-in signaling circuit. J Biol Chem 2014;289:31001-13. [Crossref] [PubMed]
- Qi L, Yu Y, Chi X, et al. Kindlin‐2 interacts with α‐actinin‐2 and β1 integrin to maintain the integrity of the Z‐disc in cardiac muscles. FEBS Lett 2015;589:2155-62. [Crossref] [PubMed]
- Hirbawi J, Bialkowska K, Bledzka KM, et al. The extreme C-terminal region of kindlin-2 is critical to its regulation of integrin activation. J Biol Chem 2017;292:14258-69. [Crossref] [PubMed]
- Plow EF, Das M, Bialkowska K, et al. Of kindlins and cancer. Discoveries 2016;4:e59. [Crossref] [PubMed]
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015;125:3335-7. [Crossref] [PubMed]
- Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544. [Crossref] [PubMed]
- Courau T, Nehar-Belaid D, Florez L, et al. TGF-β and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 2016;1:e85974. [Crossref] [PubMed]


