
Renal progenitor cells modulated by angiotensin II receptor blocker (ARB) medication and differentiation towards podocytes in anti-thy1.1 nephritis
Introduction
Mesangial proliferative glomerulonephritis (MsPGN) is an epidemic disease worldwide (1-3). The main pathological change in MsPGN is the lesion of mesangial cells (4,5). However, the depletion of podocytes in MsPGN is invariably recognized as the main cause of nephron loss, glomerulosclerosis and end-stage renal disease (ESRD), which are more serious (3,6-9).
Podocytes are highly specialized and terminally differentiated epithelial cells and are involved in many kinds of nephritis (10-14). They are irreversible and irreparable, as recognized before (3,15-17). Recent studies reported that progenitor cells, localized at the renal parietal epithelium of Bowman’s capsule (BC) (18-20), are able to potentially regenerate novel podocytes (19,21-30). Among the hierarchical subpopulations of parietal epithelial cells (PECs), renal progenitor cells, defined as cells that only express stem cell proteins and not podocyte markers, seem to be the most promising future therapeutic method for nephritis (23,31). This subset of PECs shows the potential capacity to differentiate into a transitional state expressing both stem cells and podocyte markers (32,33) and then into terminal podocytes at the vascular pole (VP) expressing only podocyte proteins (34,35). Appropriate therapeutic methods are able to modulate the differentiation of renal progenitor cells towards podocytes (36,37). MsPGN is considered an immunological disease, and angiotensin II receptor blockers (ARBs) are the primary treatment besides glucocorticoids (38,39). ARBs are common agents in the clinic due to their ability to blockade the renin-angiotensin system (RAS) and modulate varieties of cytokines (40,41). The use of ARBs in nephritis has been studied comprehensively; however, the direct cellular effects and mechanism of podocyte repopulation remain poorly understood.
The anti-thy1.1 nephritis model is typically considered the proper choice for imitating MsPGN in humans. Due to self-limited characteristics, the anti-thy1.1 nephritis model is optimal for studying recovery from MsPGN. The purpose of this study was to determine whether ARB medication attenuates experimental MsPGN by modulating the differentiation of renal progenitor cells towards podocytes and the related mechanisms.
Methods
The experimental protocol was approved by the Animal Ethics Review Committee of the Chinese People’s Liberation Army (PLA) General Hospital according to the Chinese law for the protection of animals.
Experimental anti-thy1.1 nephritis model and grouping
Male Wistar rats weighing 180–200 g were acquired from Beijing HFK Bioscience Co., Ltd., and fed at the experimental animal center of the Chinese PLA General Hospital at 24±2 °C and 50%±20% humidity. The rats were divided into the following three groups, with 24 rats within each group: (I) sham group, (II) anti-thy1.1 group (Thy-1 group) and (III) anti-thy1.1 nephritis model + losartan group (losartan group) in which losartan was given by gavage at a dose of 50 mg/kg body weight per day. On day 0, the rats in the sham group received 0.2 mL of 1× phosphate-buffered saline (pH 7.4) by tail intravenous injection. The rats in the Thy-1 group were given an intravenous injection of monoclonal anti-thy1.1 antibody (2.5 mg/kg) produced by OX-7 hybridoma cells diluted in 1× phosphate-buffered saline (pH 7.4) and were administered vehicle from days 0 to 14. The losartan group was injected with the same dose of anti-thy1.1 antibody as the Thy-1 group as above on day 0 and administered losartan potassium tablets (Merck Sharp & Dohme Corp.) dissolved in deionized water on days 0–14. Blood and urine samples were collected on days 3, 7, and 14 after modeling. In each group, eight rats were sacrificed on days 3, 7, and 14.
Blood and urine samples
Blood and urine samples were centrifuged, and the supernatants were kept at −80 °C. All samples were taken to the Biochemistry Laboratory at the Chinese PLA General Hospital. Twenty-four-hour urinary protein quantification and serum creatinine were measured using an automated biochemical analyzer.
Light microscopy
Tissues from rat kidneys were preliminarily kept in 4% paraformaldehyde and made into paraffin blocks. Sections (4 µm) were cut, deparaffinized and stained with periodic acid-Schiff (PAS). Two pathologists examined the glomerular injury of PAS staining sections for anti-thy1.1 nephritis.
Western blot analysis
Approximately 300 µL of radioimmunoprecipitation assay buffer (RIPA lysis buffer, containing 150 mM NaCl, 1.0% NP-40 or 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl at pH 8.0, protease inhibitors) was added for a ~5 mg piece of tissue. The volumes of lysis buffer were determined based on the amount of tissue present. An electric homogenizer was used to homogenize the mixture above. Protein concentrations were calculated using a bicinchoninic acid (BCA) kit (Pierce, USA). The separated protein samples (50 µL per lane) in 10% sodium dodecyl sulphate (SDS)-polyacrylamide gels were transferred to nitrocellulose membranes. We incubated the membranes overnight in 5% bovine serum albumin (BSA) and individual primary antibodies (namely, p57, WT-1, and CD24) at 4 °C. The membranes were washed three times with 1× Tris-buffered saline with Tween 20, followed by incubation with secondary antibody for 1 hour. The electrophoresis results were detected by enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, UK). The experimental procedures above were repeated at least three times.
Immunofluorescence staining
All immunofluorescence staining was performed on 4 µm tissue frozen sections from rat renal biopsy specimens (−80 °C). According to the procedure, frozen sections were preliminarily fixed in 4% paraformaldehyde for 30 minutes, processed with 0.2% Triton for 5 minutes then 5% BSA for 45 minutes, and incubated with the appropriate primary antibodies overnight as described below. Secondary antibodies, Cyanine3 (Cy3) or/and fluorescein isothiocyanate (FITC) (Beyotime Biotechnology, Shanghai, China) were incubated the next day for 1 hour to visualize the immunofluorescence results.
Single staining
To ensure that the number of podocytes increased in the Losartan group, we performed single-label immunofluorescence for p57.
Double immunofluorescence staining methods
To observe the proliferative condition of glomeruli podocytes, double staining for WT-1 and Ki-67 was performed. To detect and measure the subset of renal epithelial progenitor cells that expressed only stem cell proteins and not podocyte markers, double immunofluorescence staining for CD24 with CD133 (42) and Synaptopodin was performed. To study the pathway by which renal progenitor cells differentiate into podocytes, double staining for p-extracellular signal-regulated kinase (p-ERK) with CD133 was performed.
Confocal microscopy
Confocal microscopy was performed on 4 µm sections of frozen renal tissues by using a confocal laser fluorescence microscope (FV1000-D) (OLYMPUS, Japan). Immunofluorescence results were collected and viewed using Olympus Fluoview v4.2b software.
Primary antibodies
The following antibodies were used: anti-CD133 pAb, anti-Synaptopodin pAb, and anti-p57 Kip2 mAb (Abcam Ltd., Cambridge, UK); anti-CD24 mAb (SN3), anti-WT-1 mAb (F6), and anti-Ki-67 mAb (Santa Cruz Biotechnology, Santa Cruz, California); anti-phospho-p44/42 MAPK (ERK1/2) pAb (Thr202/Tyr204) (Cell Signaling Technology, Danvers, Massachusetts, USA).
Quantification method and statistical analysis
Positively stained podocytes were quantified in each rat using a combination of p57 and 4,6-diamino-2-phenyl indole (DAPI) staining at each time point. ImageJ software was used to measure the positive staining intensity of progenitors according to the ImageJ User Guide (version 1.50 g). Thus, we analyzed the pixel density represented by the CD133, CD24, and Synaptopodin fluorescent staining in each individual glomerulus. Shown as a percentage of the glomerular tuft area, the value (namely, CD133+/CD24+ and CD24+/Synaptopodin−) was determined by the pixel density representing the glomerular tuft area in each individual glomerulus. CD133+/pERK+ PECs were quantified as double positive CD133+pERK+ PECs/number of observed glomeruli (10 observed glomeruli each rat).
All values are reported as the mean ± standard error of at least three independent experiments. For comparisons, one-way ANOVA was used, and post hoc analyses were performed with the least significant difference test. P<0.05 was defined as a significant difference. SPSS software (version 23.0, IBM Corp., Chicago, IL, USA) was used to calculate statistical significance. GraphPad Prism software (version 7.0, San Diego, CA, USA) was used to evaluate the 50% inhibitory concentration (IC50). Adobe Illustrator CC 2019 software was used to make illustrations.
Results
Effect of losartan treatment on the anti-thy1.1 nephritis model as determined by proteinuria and pathological changes
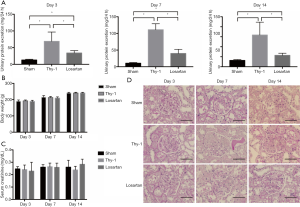
Twenty-four-hour urine for each rat was collected, and urine protein quantity was measured over 2 weeks. During the study on days 3, 7, and 14, compared with the sham group, the Thy-1 group and the losartan group presented significant persistent proteinuria (P<0.05). Additionally, compared with the Thy-1 group the losartan group showed a significant reduction in 24-hour urine protein levels (P<0.05) (day 3: 12.84±2.24 mg in the sham group, 68.95±27.72 mg in the Thy-1 group and 33.91±7.10 mg in the losartan group; day 7: 10.63±2.03 mg in the sham group, 111.23±17.66 mg in the Thy-1 group and 40.00±12.46 mg in the losartan group; day 14: 12.69±1.68 mg in the sham group, 63.58±25.63 mg in the Thy-1 group and 22.88±4.15 mg in the losartan group; Figure 1A). We examined the body weight of each rat over 2 weeks to assure the safety of losartan intake, and no significant difference among the sham, Thy-1 and losartan groups was found (Figure 1B), coinciding with the results reported by previous studies. We analyzed the serum creatinine level and found that there was no significance among the three groups (Figure 1C). Pathological changes were analyzed, and glomeruli injury was significantly attenuated in the losartan group compared with the Thy-1 group (Figure 1D).
The number of podocytes increased in the anti-thy1.1 nephritis model following losartan treatment
The number of podocytes was determined by quantifying the number of innate glomerular cells that stained for positive p57. On day 3, the number of podocytes decreased significantly in the Thy-1 and losartan groups compared with the sham group (31.4±2.3, sham group; 25.6±2.8, Thy-1 group; 27.5±2.7, losartan group; P<0.05). No difference in the number of podocytes was found between the Thy-1 and losartan groups (P>0.05). On day 7, with the development of the nephritis model, the severe depletion of podocytes persisted. Compared with the sham group, the Thy-1 group showed a significantly decreased number of podocytes (31.8±1.9 in the sham group vs. 11.6±2.3 in the Thy-1 group; P<0.05). In contrast, the number of podocytes the losartan group was increased (11.6±2.3 in the Thy-1 group vs. 23.5±4.4 in the losartan group, P<0.05). On day 14, similar to day 7, significantly more p57-positive podocytes were observed in the sham group than in the Thy-1 group (30.3±3.3 in the sham group vs. 5.9±2.2 in the Thy-1 group, P<0.05). The number of podocytes was much higher in the losartan group due to losartan treatment than in the Thy-1 group (5.9±2.2 in the Thy-1 group vs. 25.9±1.7 in the losartan group, P<0.05; Figure 2A). These data showed that compared with no treatment, losartan treatment attenuated anti-thy1.1 nephritis due to the increased number of podocytes in nephritic rats.
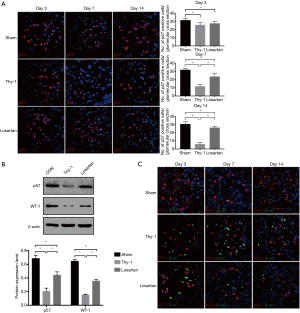
P57 and WT-1 are both proteins specifically expressed by podocytes. To demonstrate the outcome above, we performed Western blot tests for p57 and WT-1 on day 7. The protein expression of p57 and WT-1 can be measured by computer densitometry and analyzed. Western blot analysis showed that compared with the sham group, the Thy-1 and losartan groups expressed lower levels of p57 and WT-1 (p57: 0.20±0.048 sham group vs. 0.44±0.049 Thy-1 group vs. 0.68±0.049 losartan group, P<0.05) (WT-1, 0.15±0.011 sham group vs. 0.35±0.026 Thy-1 group vs. 0.64±0.026 losartan group, P<0.05). Compared with rats in the Thy-1 group, p57 and WT-1 protein expression increased significantly in the losartan group (P<0.05), consistent with the immunofluorescent results (Figure 2B). These data showed that after anti-thy1.1 antibody induction, increased protein expression of podocytes was associated with losartan treatment compared with untreated diseased rats.
Podocyte proliferation was not present in the three groups
As data shown above, the number of podocytes in the anti-thy1.1 nephritic model increased significantly in response to losartan treatment, and we sought to study how losartan treatment might lead to an increase in podocytes in rats with MsPGN. We performed double staining for WT-1 and Ki-67 to determine whether an increased number of podocytes was associated with podocyte self-proliferation. The results are shown in Figure 2C. On day 3, 7 and 14, no Ki-67+ glomerular cells were found in the sham group. Many Ki-67+ glomerular cells were detected in the Thy-1 and losartan groups due to proliferative mesangial cells, but double staining for WT-1+/Ki-67+ cells was rarely seen. Therefore, we determined that podocyte self-proliferation did not increase the number of podocytes. We sought to study PECs as progenitor cells since the increase in podocytes was not associated with podocyte self-proliferation.
Regions containing renal progenitor and transitional state cells increased following losartan treatment
To detect whether PEC regions containing potential progenitor cells, were increased with losartan treatment, we performed double staining for CD133 and CD24. Double positive staining for CD133 and CD24 was recognized as PEC regions containing progenitor cells and transitional state cells. We calculated the computer densitometry generated by ImageJ software to analyze the difference. Figure 3A, on day 3 after anti-thy1.1 antibody induction, the CD133+/CD24+ PEC region containing innate progenitors and transitional state cells was not significantly different between the Thy-1 group and the sham group (P>0.05), but a larger CD133+/CD24+ region was detected in the losartan group (P<0.05) (5.868%±0.629% in the sham group, 6.813%±0.760% in the Thy-1 group and 11.952%±1.957%/glomerular cross section in the losartan group). On day 7 and 14, an enlarged CD133+/CD24+ PEC region was found in the Thy-1 and losartan groups compared with the sham group (P<0.05) (day 7: 5.606%±1.595%/glomerular cross section in the sham group, 11.054%±1.742%/glomerular cross section in the Thy-1 group and 14.035%±1.883%/glomerular cross section in the losartan group; day 14: 5.162%±1.078%/glomerular cross section in the sham group, 8.710%±1.802%/glomerular cross section in the Thy-1 group and 12.065%±2.122%/glomerular cross section in the losartan group), and the region was detected in the losartan group was larger than that detected in the Thy-1 group (P<0.05).
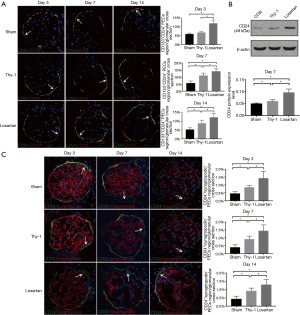
As shown in Figure 3B, we also analyzed the day 7 CD24 Western blot results. CD24 protein expression was higher in the Thy-1 and losartan groups than in the sham group (0.050±0.003 in the sham group vs. 0.060±0.006 in the Thy-1 group and 0.096±0.015 in the losartan group, P<0.05), and CD24 expression increased significantly in the losartan group after losartan treatment compared with the Thy-1 group (P<0.05).
The area of renal progenitor cells in the PEC regions extended in response to losartan treatment
According to previous studies, renal progenitor cells are defined as renal PECs expressing only stem cell proteins without podocyte markers. CD24+/Synaptopodin- staining was performed to locate renal innate progenitors. In this study, we found that the area of renal progenitor cells in the PEC region was significantly increased in the Thy-1 and losartan groups compared with the sham group (P<0.05), and the range of CD24+/Synaptopodin− was significantly increased in the losartan group compared with the Thy-1 group (P<0.05) (day 3: 0.45%±0.13%/glomerular cross section in the sham group, 0.84%±0.12%/glomerular cross section in the Thy-1 group and 1.42%±0.44%/glomerular cross section in the losartan group; day 7: 0.39%±0.17%/glomerular cross section in the sham group, 0.91%±0.20%/glomerular cross section in the Thy-1 group and 1.44%±0.39%/glomerular cross section in the losartan group; day 14: 0.42%±0.17%/glomerular cross section in the sham group, 0.91%±0.18%/glomerular cross section in the Thy-1 group and 1.28%±0.31%/glomerular cross section in the losartan group; Figure 3C).
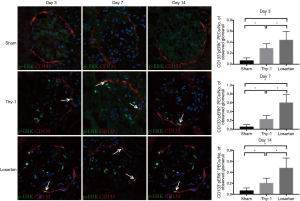
P-ERK1/2 signaling pathway was augmented in losartan-treated rats with experimental MsPGN
The p-ERK signaling pathway is involved in the regulation of the growth, division, development, differentiation and proliferation of several epithelial cells (43). We performed double immunofluorescent staining for CD133 and p-ERK1/2 to detect the ERK pathway. Double positive CD133+/p-ERK+ PECs were defined as positive renal PECs. In this study, we found that the p-ERK1/2 signaling pathway participated in the differentiation of renal progenitor cells into podocytes. Additionally, the p-ERK1/2 pathway can be regulated by losartan administration (day 3: 0.063±0.052 in the sham group, 0.288±0.084 in the Thy-1 group and 0.438±0.160 in the losartan group; day 7: 0.050±0.054 in the sham group, 0.225±0.089 in the Thy-1 group and 0.600±0.185 in the losartan group; day 14: 0.063±0.052 in the sham group, 0.200±0.093 in the Thy-1 group and 0.475±0.183 in the losartan group; Figure 4).
Discussion
MsPGN is accepted as immunocomplex-induced nephritis, and pathological studies have also confirmed that MsPGN is mostly caused by systemic immune problems (44,45). ARBs, as safe and effective medications, are extensively used by MsPGN patients to treat proteinuria. The benefits of ARBs for MsPGN patients with proteinuria have been described adequately in both basic and clinical studies. The number of podocytes increased significantly following ARB treatment for MsPGN in both animals and humans, as previously reported. However, in contrast to corticosteroids, ARBs show insufficient effects on immune disorders and limited ability to prevent podocytes from undergoing apoptosis, which prompted us to focus on its direct cellular effect. In this study, we demonstrate that the depletion of podocytes occurs rapidly in rats with experimental MsPGN after anti-thy1.1 antibody induction, and the number of podocytes can be increased by losartan administration due to the significantly increased progenitor cell domain.
The vascular barrier formed by podocytes is functionally critical to the nephron. As highly specialized and terminally differentiated epithelial cells, podocytes lack the ability to self-repopulate (46,47). To our knowledge, as long as the depletion of podocytes is moderate, glomeruli restitution is possible. However, if podocyte loss becomes severe and the proportion of involved glomeruli increases, globally sclerotic and nonfiltering glomeruli can be found, corresponding with persistent proteinuria and a measurable reduction in the glomerular filtration rate (3,14). Therefore, podocyte injury is recognized as the initiating and primary factor in the development of various types of nephritis (15). During MsPGN development, persistent proteinuria indicates severely increased depletion of podocytes. How might losartan help to repair glomeruli in MsPGN? A rat model of MsPGN was used in this study. The experimental rat model induced with the anti-thy1.1 antibody is the typical nephritic model for human MsPGN. As reported before, due to its self-limited characteristics, this model is optimal for studying recovery from MsPGN and whether the proliferation of renal progenitor cells helps to replace damaged podocytes.
First, we selected p57, which is restricted to podocytes in glomeruli, to determine the number of podocytes. Throughout the progression of anti-thy1.1 nephritis, we analyzed the number of podocytes on days 3, 7, and 14. On day 3 after disease induction, the number of podocytes started to decline significantly and proteinuria was detected. With the development of anti-thy1.1 nephritis, the depletion of podocytes became the most severe on day 7, consistent with relevant pathological manifestations and marked proteinuria. On day 14, the number of podocytes was partially restored, but glomerular scarring remained, and proteinuria persisted. A significantly increased number of podocytes was found in the losartan group compared with the Thy-1 group. Moreover, an increase in podocytes directly correlated with reduced proteinuria, as reported in previous pathological studies.
WT-1/Ki-67 double staining was performed to demonstrate whether the increased number of podocytes was due to self-proliferation. In this study, we found many proliferative cells along the intraglomerular mesangial region in the Thy-1 and losartan groups, as expected. However, WT-1+/Ki-67+ cells were rarely found in the three groups, indicating that podocyte proliferation was limited. As terminally differentiated epithelial cells, podocytes cannot adequately replace any decrease in cell number due to the lack of the necessary cell cycle machinery. Therefore, we evaluated the effect of podocyte repopulation on cellular effects, especially on renal progenitor cells.
To describe the proliferation of progenitor cells, CD133/CD24 and CD24/Synaptopodin double staining were performed. Innate renal progenitor cells were defined as cells that only expressed stem cell protein and not podocyte markers and that resided along BC. CD133+/CD24+ staining represented the area containing progenitor cells and transitional state cells, while CD24+/Synaptopodin− staining labeled renal progenitor cells. In this study, the CD133+/CD24+ area (staining along BC) was significantly augmented in the losartan-treated group compared with the untreated Thy-1 group. Likewise, a more enlarged CD24+/Synaptopodin− area was detected. Among the hierarchical subpopulations of PECs, renal progenitor cells express stem cell markers, and transitional state cells express both stem cells and podocyte markers, which then terminally differentiate into the podocytes. According to the study led by Shankland (31), only stem cell marker+/podocyte marker− PECs displayed the potential to regenerate podocytes and to improve glomerular injury, and stem cell marker+/podocyte marker+ PECs were not able to improve kidney injury and rarely generated podocytes due to their limited engraftment capacity and lack of self-renewal potential (3,15,16,48). In the CD24/Synaptopodin staining study, significantly augmented PECs expressing stem cell markers, namely, renal progenitor cells, were found in the losartan group compared with the Thy-1 group. This finding indicated that losartan, as an ARB, is potentially helpful in regulating the differentiation of renal progenitor cells into podocytes to attenuate anti-thy1.1 nephritis.
We did not discover the precise mechanism that regulates the differentiation of progenitor cells into podocytes due to complex and not one-fit-all signal pathways. Our study focused on the p-ERK signaling pathway because ERK is a member of the mitogen-activated protein kinase (MAPK) family and is involved in the regulation of the growth, division, development, differentiation and proliferation of several epithelial cells. We found that several PECs along BC co-expressed CD133+/p-ERK+ in the Thy-1 group. After losartan administration, additional CD133+/p-ERK+ PECs were detected. All the results suggested that the p-ERK signaling pathway participated in PEC progenitor cell differentiation into podocytes and that losartan was able to regulate this pathway. However, this research method was limited, and we could not prove that the p-ERK pathway primarily underlied the effect.
Conclusions
Finally, in summary, losartan could attenuate the anti-thy1.1 nephritic rat model by increasing the number of podocytes, which was correlated with reduced proteinuria and the alleviation of pathological changes. We detected an increase in progenitor cells after losartan administration, which was important in podocyte repopulation. The p-ERK1/2 signaling pathway participated in the differentiation of renal progenitor PECs into podocytes, which will be important for the treatment of kidney disease in the future. We considered renal progenitor cells, namely, PECs, to be another important factor for saving injured kidneys.
Acknowledgments
Funding: All equipment and drugs mentioned in this paper were funded by National Natural Science Foundation of China (No. 81330019).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experimental protocol was approved by the Animal Ethics Review Committee of the Chinese People’s Liberation Army (PLA) General Hospital according to the Chinese law for the protection of animals (No. 2017-X14-60).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brück K, Stel VS, Gambaro G, et al. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol 2016;27:2135-47. [Crossref] [PubMed]
- Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019;322:1294-304. [Crossref] [PubMed]
- Trimarchi H, Barratt J, Cattran DC, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017;91:1014-21. [Crossref] [PubMed]
- Arias LF, Taborda-Murillo A. Mesangial proliferative glomerulonephritis: A glomerular disease or a non-specific morphological change? Nephrology (Carlton) 2017;22:575. [Crossref] [PubMed]
- Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers 2016;2:16001. [Crossref] [PubMed]
- Calizo RC, Bhattacharya S, van Hasselt JGC, et al. Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat Commun 2019;10:2061. [Crossref] [PubMed]
- Schell C, Huber TB. The Evolving Complexity of the Podocyte Cytoskeleton. J Am Soc Nephrol 2017;28:3166-74. [Crossref] [PubMed]
- Nihalani D, Solanki AK, Arif E, et al. Disruption of the exocyst induces podocyte loss and dysfunction. J Biol Chem 2019;294:10104-19. [Crossref] [PubMed]
- Trimarchi H, Coppo R. Podocytopathy in the mesangial proliferative immuno-globulin A nephropathy: new insights into the mechanisms of damage and progression. Nephrol Dial Transplant 2019;34:1280-5. [Crossref] [PubMed]
- Allison SJ. Podocyte biology: The podocyte adhesome. Nat Rev Nephrol 2017;13:445. [Crossref] [PubMed]
- Inoue K, Gan G, Ciarleglio M, et al. Podocyte histone deacetylase activity regulates murine and human glomerular diseases. J Clin Invest 2019;129:1295-313. [Crossref] [PubMed]
- Nagata M. Podocyte injury and its consequences. Kidney Int 2016;89:1221-30. [Crossref] [PubMed]
- Qi YY, Zhou XJ, Cheng FJ, et al. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-alpha in lupus nephritis. Ann Rheum Dis 2018;77:1799-809. [Crossref] [PubMed]
- Saleem MA. Molecular stratification of idiopathic nephrotic syndrome. Nat Rev Nephrol 2019;15:750-65. [Crossref] [PubMed]
- Miyazaki Y, Shimizu A, Ichikawa I, et al. Mice are unable to endogenously regenerate podocytes during the repair of immunotoxin-induced glomerular injury. Nephrol Dial Transplant 2014;29:1005-12. [Crossref] [PubMed]
- Grahammer F, Wanner N, Huber TB. Podocyte regeneration: who can become a podocyte? Am J Pathol 2013;183:333-5. [Crossref] [PubMed]
- Wang M. Lipids mediate podocyte damage. Nat Rev Nephrol 2019;15:594. [PubMed]
- Andeen NK, Nguyen TQ, Steegh F, et al. The phenotypes of podocytes and parietal epithelial cells may overlap in diabetic nephropathy. Kidney Int 2015;88:1099-107. [Crossref] [PubMed]
- Lasagni L, Angelotti ML, Ronconi E, et al. Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Re-mission and Can Be Pharmacologically Enhanced. Stem Cell Reports 2015;5:248-63. [Crossref] [PubMed]
- Kaverina NV, Eng DG, Schneider RR, et al. Partial podocyte replenishment in ex-perimental FSGS derives from nonpodocyte sources. Am J Physiol Renal Physiol 2016;310:F1397-413. [Crossref] [PubMed]
- Berger K, Schulte K, Boor P, et al. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol 2014;25:693-705. [Crossref] [PubMed]
- Shankland SJ, Pippin JW, Duffield JS. Progenitor cells and podocyte regeneration. Semin Nephrol 2014;34:418-28. [Crossref] [PubMed]
- Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 2013;9:137-46. [Crossref] [PubMed]
- Lim BJ, Yang JW, Do WS, et al. Pathogenesis of Focal Segmental Glomerulosclerosis. J Pathol Transl Med 2016;50:405-10. [Crossref] [PubMed]
- Endlich N, Kliewe F, Kindt F, et al. The transcription factor Dach1 is essential for podocyte function. J Cell Mol Med 2018;22:2656-69. [Crossref] [PubMed]
- Roeder SS, Stefanska A, Eng DG, et al. Changes in glomerular parietal epithelial cells in mouse kidneys with advanced age. Am J Physiol Renal Physiol 2015;309:F164-78. [Crossref] [PubMed]
- Manonelles A, Guiteras R, Melilli E, et al. The Presence of Urinary Renal Progenitor Cells in Stable Kidney Transplant Recipients Anticipates Allograft Deterioration. Front Physiol 2018;9:1412. [Crossref] [PubMed]
- Kietzmann L, Guhr SS, Meyer TN, et al. MicroRNA-193a Regulates the Transdifferentiation of Human Parietal Epithelial Cells toward a Podocyte Phenotype. J Am Soc Nephrol 2015;26:1389-401. [Crossref] [PubMed]
- Moeller MJ, Tharaux PL. Cellular regeneration of podocytes from parietal cells: the debate is still open. Kidney Int 2019;96:542-44. [Crossref] [PubMed]
- Shankland SJ, Freedman BS, Pippin JW. Can podocytes be regenerated in adults? Curr Opin Nephrol Hypertens 2017;26:154-64. [Crossref] [PubMed]
- Shankland SJ, Smeets B, Pippin JW, et al. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 2014;10:158-73. [Crossref] [PubMed]
- Kaverina NV, Eng DG, Freedman BS, et al. Dual lineage tracing shows that glomerular parietal epithelial cells can transdifferentiate toward the adult podocyte fate. Kidney Int 2019;96:597-611. [Crossref] [PubMed]
- Hayashi A, Okamoto T, Yamazaki T, et al. CD44-Positive Glomerular Parietal Epithelial Cells in a Mouse Model of Calcineurin Inhibitors-Induced Nephrotoxicity. Nephron 2019;142:71-81. [Crossref] [PubMed]
- Kuppe C, Leuchtle K, Wagner A, et al. Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney Int 2019;96:80-93. [Crossref] [PubMed]
- Eng DG, Sunseri MW, Kaverina NV, et al. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int 2015;88:999-1012. [Crossref] [PubMed]
- D'Agati VD, Shankland SJ. Recognizing diversity in parietal epithelial cells. Kidney Int 2019;96:16-9. [Crossref] [PubMed]
- Zhang J, Pippin JW, Krofft RD, et al. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 2013;304:F1375-89. [Crossref] [PubMed]
- Pozzi C. Treatment of IgA nephropathy. J Nephrol 2016;29:21-5. [Crossref] [PubMed]
- Holdsworth SR, Kitching AR. Progress in mechanisms and therapy for immunological kidney disease. Nat Rev Nephrol 2018;14:76-78. [Crossref] [PubMed]
- Rauen T, Eitner F, Fitzner C, et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N Engl J Med 2015;373:2225-36. [Crossref] [PubMed]
- Xiao C, Zhou Q, Li X, et al. Losartan and Dexamethasone may inhibit chemotaxis to reduce the infiltration of Th22 cells in IgA nephropathy. Int Immunopharmacol 2017;42:203-8. [Crossref] [PubMed]
- Ehsani E, Shekarchian S, Baharvand H, et al. Improved differentiation of human enriched CD133+CD24+ renal progenitor cells derived from embryonic stem cell with embryonic mouse kidney-derived mesenchymal stem cells co-culture. Differentiation 2019;109:1-8. [Crossref] [PubMed]
- Kurtzeborn K, Kwon HN, Kuure S. MAPK/ERK Signaling in Regulation of Renal Differentiation. Int J Mol Sci 2019;20:1779. [Crossref] [PubMed]
- Lu Y, Mei Y, Chen L, et al. The role of transcriptional factor D-site-binding protein in circadian CCL2 gene expression in anti-Thy1 nephritis. Cell Mol Immunol 2019;16:735-45. [Crossref] [PubMed]
- Liu P, Lassén E, Nair V, et al. Transcriptomic and Proteomic Profiling Provides Insight into Mesangial Cell Function in IgA Nephropathy. J Am Soc Nephrol 2017;28:2961-72. [Crossref] [PubMed]
- Liu M, Liang K, Zhen J, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 2017;8:413. [Crossref] [PubMed]
- Assady S, Wanner N, Skorecki KL, et al. New Insights into Podocyte Biology in Glomerular Health and Disease. J Am Soc Nephrol 2017;28:1707-15. [Crossref] [PubMed]
- Puelles VG, Moeller MJ. Postnatal podocyte gain: Is the jury still out? Semin Cell Dev Biol 2019;91:147-52. [Crossref] [PubMed]


