
To combine or not to combine: anti-vascular endothelial growth factor therapies in EGFR mutation positive non-small cell lung cancer
The concomitant use of vascular endothelial growth factor (VEGF) inhibitors with cytotoxic chemotherapy reportedly enhances treatment efficacy in patients with non-small cell lung cancer (NSCLC). The anti-angiogenic monoclonal antibody bevacizumab targets the VEGF signaling pathway and has been shown to provide additional efficacy when used in combination with first-line platinum-based chemotherapy in several trials for non-squamous NSCLC (1-3). The combination of docetaxel and ramucirumab, an anti-VEGFR2 antibody, has been established as a standard treatment in patients with refractory NSCLC who experienced progression after a first-line platinum-based combination (4).
In addition to cytotoxic agents, the combination of erlotinib and bevacizumab has the potential to prolong progression-free survival (PFS) in unselected populations of patients with NSCLC (5,6). In a subgroup analysis of EGFR-mutation-positive participants in the phase 3 BeTa study examining second-line treatments for NSCLC (12 patients treated with erlotinib and bevacizumab and 18 with erlotinib alone), the median PFS period for patients with EGFR-mutation-positive disease who were treated with erlotinib plus bevacizumab was substantially higher than that in patients treated with erlotinib alone (17.1 vs. 9.7 months) (5).
About 10 years ago, first-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib and erlotinib were shown, for the first time, to exert an efficacy that was superior to that of cytotoxic anticancer agents in patients with NSCLC harboring an EGFR mutation.
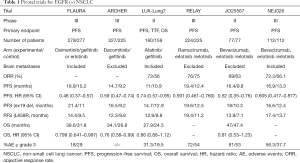
When PFS was examined in the LUX-Lung7 trial, the second-generation EGFR-TKI afatinib was shown to have a hazard ratio of 0.74 (95% CI, 0.57–0.95), compared with gefitinib (7). However, the median PFS periods were 11.0 and 10.9 months, respectively. In 2018, the ARCHER1050 trial reported the results of a comparison between dacomitinib, another second-generation EGFR-TKI, and gefitinib (8). In this study, dacomitinib was more effective than gefitinib in terms of both PFS (HR, 0.59; 95% CI, 0.47–0.74) and overall survival (OS) (HR, 0.76; 95% CI, 0.58–0.99).
Osimertinib, a third-generation EGFR-TKI, is an effective drug against the T790M mutation, which is the main resistance mechanism. The FLAURA trial was the first phase III trial comparing osimertinib and first-generation EGFR-TKIs (gefitinib, erlotinib) in patients with NSCLC without previous treatment (9). Patients assigned to first-generation EGFR-TKIs were also offered osimertinib as a crossover treatment after disease progression. Osimertinib showed better results than the first-generation EGFR-TKIs in terms of both PFS (HR, 0.46; 95% CI, 0.37–0.57) and OS (HR, 0.799; 95% CI, 0.641–0.997).
On the other hand, attempts to use an EGFR-TKI and an angiogenesis inhibitor in combination have been made for the treatment of patients with EGFR-mutation-positive NSCLC. The JO25567 trial was a randomized phase II trial comparing erlotinib with erlotinib + bevacizumab (10). The median PFS period for erlotinib was 9.8 months, compared with 16.4 months in the combination group (HR, 0.52; 95% CI, 0.35–0.76), showing the significant efficacy of using a combination of erlotinib and bevacizumab. The NEJ026 trial was the first phase III trial using EGFR-TKIs and angiogenesis inhibitors (11). As described above, the bevacizumab combination group had a statistically significant longer PFS period, with an HR of 0.605 (95% CI, 0.417–0.877). In addition, the results of the RELAY study, a phase three study using ramucirumab (another angiogenesis inhibitor), have also been reported (12). In this study, the PFS was clearly prolonged, with an HR of 0.591 (95% CI, 0.461–0.760) in the combined treatment group that included the angiogenesis inhibitor. In the NEJ026 and RELAY studies, OS analyses were planned but have not yet been reported.
In summary, osimertinib, dacomitinib, erlotinib + bevacizumab, and erlotinib + ramucirumab all showed significant results in terms of PFS, which was the primary endpoint of the studies. Among them, the efficacies of osimertinib and dacomitinib have also been confirmed in terms of OS (Table 1).
Full table
An improvement in drug delivery is one possible mechanism explaining the synergistic efficacy provided by anti-VEGF inhibitors. Several studies have reported that bevacizumab changes the tumor vessel physiology, resulting in an increased intra-tumoral concentration of drugs (13,14). Concerning the resistance mechanism of EGFR-TKIs in patients with EGFR-mutated NSCLC, one study suggested that patients on lower doses of EGFR-TKIs tend to develop treatment resistance earlier than those who receive higher doses (15). When EGFR-TKIs are used in combination with anti-VEGF inhibitors, the higher intra-tumoral concentration of EGFR-TKIs could delay the appearance of resistant cells. In addition to the increased concentration provided by improved drug delivery, VEGF inhibition might be effective in tumors harboring resistance mutations that act against EGFR-TKIs. In preclinical studies, blocking the VEGF signaling pathway enabled the T790M resistance mutation to be overcome (16,17).
There are some obvious drawbacks to combining anti-VEGF and EGFR-TKIs. This combination cannot be used in patients with contraindications to anti-VEGF, such as macrovascular invasion or a history of hemoptysis. Furthermore, the need for a 3-week infusion schedule for bevacizumab and a 2-week infusion schedule for ramucirumab is inconvenient for the patients. Finally, anti-VEGF and EGFR-TKI combination therapy clearly increases the risk of adverse events, compared with osimertinib.
The combined use of anti-VEGF and EGFR-TKIs has shown results that exceed those of dacomitinib and afatinib in terms of PFS, and the median PFS was comparable to that achievable using osimertinib. Unfortunately, patients with T790M resistance mutations cannot be identified prior to their first treatment; however, if anti-VEGF and EGFR-TKIs are used in the initial treatment and T790M mutations are subsequently identified, osimertinib can be used and a very long OS can be expected. In a subset analysis, EGFR-TKI monotherapy, including osimertinib, tended to result in a poor PFS in patients with L858R mutation-positive disease; however, anti-VEGF and EGFR-TKI combination therapy was shown to be effective regardless of the mutation type (Table 1). In addition, patients with brain metastases and pleural effusion were also registered in the NEJ026 study, and a favorable PFS was shown in the anti-VEGF combination group in these subsets (11). These findings suggest that combination therapy with anti-VEGF and EGFR-TKI confers important advantages, and the next step will be to examine the effects of combination therapy with bevacizumab or ramucirumab and osimertinib [TORG1833 (JapicCTI-184146), UMIN000030142, NCT03909334, WJOG9717L (UMIN000030206)].
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.01.66). HH reports grants and personal fees from BMS, grants and personal fees from MSD, grants and personal fees from Chugai, grants and personal fees from Taiho, grants and personal fees from Astra Zeneca, grants from Astellas, grants from Merck Serono, grants from Genomic Health, grants and personal fees from Lilly, grants and personal fees from Ono, outside the submitted work.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012;76:362-7. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846-54. [Crossref] [PubMed]
- Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743-50. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244-50. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-35. [Crossref] [PubMed]
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. [Crossref] [PubMed]
- Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 2003;88:1979-86. [Crossref] [PubMed]
- Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res 2007;13:3942-50. [Crossref] [PubMed]
- Hayakawa H, Ichihara E, Ohashi K, et al. Lower gefitinib dose led to earlier resistance acquisition before emergence of T790M mutation in epidermal growth factor receptor-mutated lung cancer model. Cancer Sci 2013;104:1440-6. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Ichihara E, Ohashi K, Takigawa N, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res 2009;69:5091-8. [Crossref] [PubMed]


