
Early removal of chest tubes leads to better short-term outcome after video-assisted thoracoscopic surgery lung resection
Introduction
Recently, advances in minimally-invasive techniques for thoracic surgery, including lobectomy and wedge-resection by video-assisted thoracoscopic surgery (VATS), have accelerated the post-operative recovery of patients by decreasing pain after surgery and reducing the incidence of complications, allowing surgeons to remove drainage tubes as soon as possible (1,2).
For patients after thoracic surgery, insufficient re-dilation of the residual lung is one of the most important factors after lobectomy, and may lead to post-operative hemothorax and persistent air leakage. In conventional surgical procedures, with patients who underwent lobectomy or wedge resection by VATS, surgeons would like to use two drains—one at the tip of the pleura, and the other posterior and basal (3). However, a chest tube in the pleural cavity can cause various complications including increasing the degree of pain and the risk of infection, thus prolonging the hospital stay (4). The most frequent complication following a pulmonary resection is an alveolar air leak. In patients who suffered from an alveolar air leak after thoracic surgery this is the most important determinant of length of hospital stay (5,6).
In light of earlier findings, some surgeons believed that early removal of a chest tube reduces length of hospital stay and decreases complications after VATS lobectomy with no air leakage when drainage is 500 mL/day or less (7). The purpose of our study was to retrospectively analyze the association between early removal of a chest tube and length of hospital stay in patients treated by lobectomy.
Methods
Study design and patient inclusion
All patients with lung tumors who were continuously hospitalized in the First Affiliated Hospital of Guangzhou Medical University from April 2018 through March 2019 were retrospectively identified and collected. All initial patients were evaluated to select patients who were likely to undergo thoracic surgery including wedge-resection and lobectomy by VATS when they were admitted to our center, the criteria for admission were as follows: no cardiopulmonary insufficiency such as coronary artery abnormality, no underlying disease such as diabetes coagulation abnormality or liver cirrhosis, no obvious surgical contraindications, no metastatic tumors in any other organs by positron emission tomography (PET) or computed tomography (CT). Exclusion criteria: patients who changed surgical procedure during operation, patients who were found to have total pleural adhesion during the operation, patients with malignant tumors with obvious invasion to the adjacent organs such as reconstructive vascular operation, patients who did not have a chest tube placed after surgery.
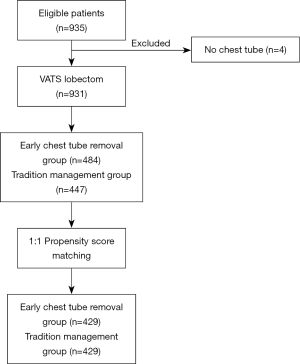
This was a retrospective study. A total of 931 patients were divided into two groups: the experimental group and the control group, and whether the chest tube was removed early or not was determined according to the drainage time. For the purposes of this study, early removal was defined as within the first 48 hours. In the experimental group, the chest tube was removed within 48 hours after VATS lung resection (drainage time ≤2 days). In the control group, the chest tube remained in place inside the thoracic cavity for more than 48 hours (drainage time >2 days) (Figure 1).
Surgical procedures
Under spontaneous respiration anesthesia or intubation anesthesia, wedge-resection and lobectomy were performed with one or two incisions without traction of surrounding muscle tissue, following the procedure described in previous studies (8). However, normally, two incisions were routinely chosen in our hospital. Sometimes, some of the surgeons preferred to use the uniportal technique as their first choice in our center. Usually, two incisions were selected, at the fourth and sixth intercostal or fifth and seventh intercostal spaces according to the tumor position. All patients in this study accepted the intercostal block which is a type of anesthesia and the blocking drug was selected as lidocaine, the pharmacodynamic effect of which continued for 3–5 hours to control the breathing. Next, all surgeons would block the phrenic nerve using 8–10 mL of lidocaine to reduce the movement of the mediastinum. Similar surgical procedures were performed by different surgeons in each department. More and more detailed surgical procedures have been described in other articles (9). After pneumonectomy, complete thoracic lymph node dissection was performed in patients with non-small cell lung cancer (10-12). Following adequate hemostasis, a chest tube of the same size was placed in the remaining space in the thoracic cavity through the wound on the mid-axillary line (13). The incision was closed. In all cases, each chest tube was connected to a water-sealed bottle until the chest tube was removed, to reduce the size of the wound cavity. It was worth noting that the chest tube and the water-sealed bottle were removed together. The volume of drainage was recorded by residents or nurses. All patients in this study accepted suction immediately after the end of surgery to extract residual gas and liquid in the thoracic cavity. In some cases, patients were kept on suction (negative pressure 10 cmH2O) by some surgeons for several hours after surgery.
Chest tube protocol
All patients in this study were treated by lobectomy and wedge-resection using minimally invasive techniques by VATS. The surgical procedures were as previously described (5,11,12). The surgeon used suction to remove residual gas or liquid from the thoracic cavity using a negative pressure drainage device within the recovery room. Then, the surgeon confirmed that the lungs were completely expanded before the patients were taken back to the ward. The volume of fluid drained from the chest tube was recorded every morning at nine o’clock by residents in our center. At the same time, the color of the liquid was recorded by nurses. The resident recorded any complications such as infection after surgery, large pleural effusion or pain during hospitalization.
Postoperative management
There were some clinical criteria for early removal of the chest tube, as follows: (I) no air leakage from the thoracic cavity; (II) the absence of purulent pleural effusion or chylous; (III) patients without atelectasis on a chest roentgenogram the first day after operation. The chest tube was removed on the day after VATS lung resection when patients fulfilled those criteria; we did not need to consider the drainage volume. All patients from whom the chest tube was removed underwent chest X-ray within 24 hours to estimate lung expansion.
Postoperative data were collected from all patients during hospitalization, which consisted of daily assessment of the drainage volume from the thoracic cavity until the chest tube was removed (performed every morning), no obvious air leakage, and complete expansion of the lung determined by chest X-ray. Drainage time and postoperative hospital stay were recorded until the patient recovered and left the hospital. As well as intraoperative bleed, tumor diameter (the pathological specimen after surgery), total drainage, anesthesia [including intubated VATS (I-VATS) and non-intubated VATS (NI-VATS)] and number of chest tubes were recorded during hospitalization. Postoperative complications (including re-operation, lung infection, pleural effusion, no complications, others or re-placing of a chest tube) were also recorded. Other complications included subcutaneous emphysema, pneumothorax caused by the clinical procedure, unexplained persistent air leakage, pneumonia affecting the length of stay in hospital and many more. Chest X-rays were performed after the chest tube was removed. All procedures were used to determine the amount of fluid in the pleural space after the chest tube was removed. In each patient it was necessary to empty the accumulated fluid by doing a thoracentesis when the fluid in the pleural cavity filled more than 10–30% of the pleural cavity, which could affect the patient’s quality of life. In addition, patients with pneumothorax and recurrent effusion were identified by patient symptoms, chest X-rays and even CT. All patients’ conditions were recorded in this study by nurses and residents truthfully.
Statistical analysis
The subjects were divided into two groups according to the drainage time before the chest tube was removed. The variables that were selected included preoperative data and postoperative variables.
Continuous data are expressed as mean and standard deviation (SD), and were analyzed by 2-sample Student’s t-test of independent data. Categorical variables are given as a count and percentage of patients and compared using the χ2 or Fisher’s exact test. All tests were 2-sided, with an A-level of 0.05. SPSS software was used for all statistical evaluations.
Results
Baseline data
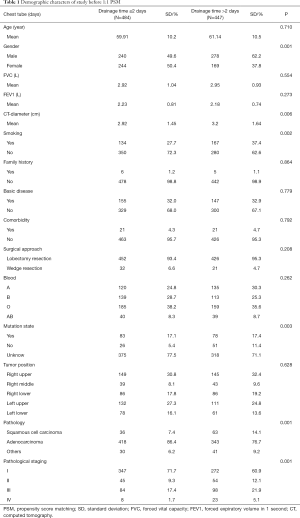
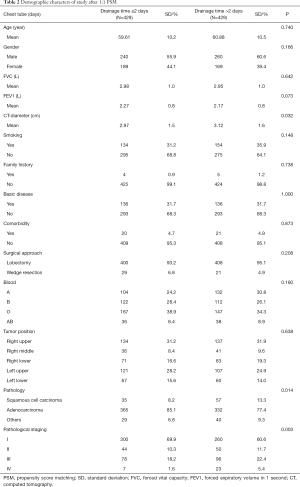
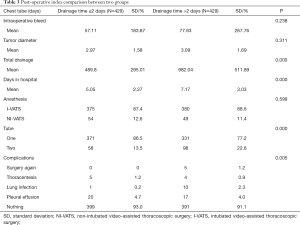
Between April 2018 and March 2019, a total of 935 consecutive patients underwent lobectomy and wedge-resection. Four of the patients were excluded from the research because a chest tube was not inserted after the surgery, according to the exclusion criteria. The remaining 931 patients were eventually included in this analysis. The mean age at surgery was 59.91±10.2 years for the experimental group and 61.14±10.5 years for the control group. The baseline data of all patients are shown in Table 1. After 1:1 propensity score matching (PSM), 858 (429 vs. 429) patients remained in the analysis, and the baseline demographic and clinical variables were perfectly balanced between the experimental group and the control group. In total, 697 patients (81.24%) were diagnosed with adenocarcinoma and 654 (76.22%) with early-stage tumor including stage I and stage II. The experimental group and control group were comparable with regard to mean age at surgery, forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), family history, basic disease, comorbidity, surgical approach, blood and tumor position, with no significant difference (P>0.05). The baseline data of all patients for both experimental group and control group are shown in Table 2.
Full table
Full table
Postoperative complications and hospital stay
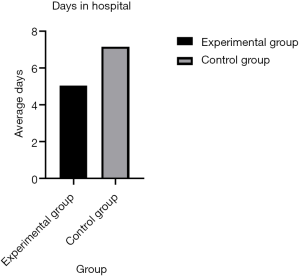
All postoperative complications were recorded by residents during hospitalization. For the hospital stay, the experimental group had a significantly shorter length of stay than the traditional management group (5.05±2.27 vs. 7.17±3.03; P<0.001) (Figure 2).
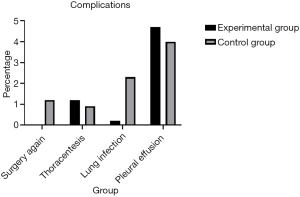
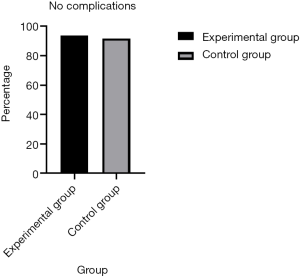
Regarding complications, a significant decrease in the number of patients developing lung infections after removal of the chest tube was observed in the early removal group compared with the traditional management group (0.2% vs. 2.3%, P=0.005). There were more patients without complications in the early removal group compared to the traditional management group (93.0% vs. 91.1%, P=0.005) (Figure 3). Furthermore, 5 (1.2%) patients required repeated surgery (P=0.005). However, both pleural effusion and thoracentesis showed a significant increase in patient number in the early removal group compared with the traditional management group (4.7% vs. 4.0%, 1.2% vs. 0.9%; P=0.005) (Figure 4). Perioperative data for both experimental group and control group are presented in Table 3.
Full table
Discussion
Minimally-invasive surgical techniques, particularly thoracoscopic surgery, have been performed since 1990 (14). On this background, a number of disciplines have also made great progress and development, and these subjects included anesthesia, pain control, perioperative support and many other areas (13). In some patients undergoing elective surgery, the concept of rapid recovery from surgery combines the advantages of nursing operation and perioperative period. The methods include different methods of anesthesia, advances in minimally-invasive surgery, reasonable pain management and active postoperative rehabilitation (15).
Recently, for patients undergoing surgery, the fast-tracking protocols, cost containment measures, and cost containment measures have been discussed (16-18). Both Robert JM and Robert JC reported that by using a fast-tracking protocol combined with VATS lobectomy, surgery can be conducted with minimal complications, resulting in a shorter length of stay in hospital, and decreased costs (19,20). There is no doubt that with the development of minimally-invasive surgical techniques, most surgeons are interested not only in the management of chest tubes, but also in specific procedures for rapid recovery after surgery. In addition, in safe surroundings, most surgeons could remove a chest tube as soon as possible after surgery because it can significantly shorten the length of hospital stay and thus reduce costs, as stated in a previous study (19).
From a theoretical point of view, advances in thoracic surgical techniques have reduced postoperative pain; currently therefore, the degree of pain caused by the chest tube is even more prominent (21). Many surgeons believe that chest tubes are also a risk factor for pain. All thoracic surgeons try to reduce the degree of pain after surgery in patients using a variety of treatments. Most thoracic surgeons reduce pain by improving medical procedures and nursing level. The problem of postoperative pain has gradually attracted the awareness of surgeons. Recently, the safety of early removal of the chest tube has been discussed and reported in several published studies (19,20,22,23).
At present, there is no detailed statement on the optimal time to remove the chest tube according to the drainage volume after VATS lung resection, and at the same time, there have been no relevant studies of this problem (24). Clinically, most surgeons will remove the chest tube when they consider it suitable, according to the clinical criteria in our center. Most surgeons regard drainage volume from the chest tube before removal as evidence for the removal of the chest tube (7,19,25). They suggest that a threshold of 100–250 milliliters per day is a safe threshold but this remains a controversial point of view. Thoracentesis was performed when the amount of pleural effusions in the pleural cavity, and the proportion of patients requiring intervention after the chest tube was removed. Several experts have reported that the threshold for safe removal of chest tubes is as high as 300 (19), 400 (25), or 500 mL per day (7), while some surgeons consider it safe even with an unlimited drainage threshold as long as there are no obvious complications such as air leakage or chylothorax (9). Many surgeons regard a drainage volume of 100–250 mL per day as an important factor in removing chest tubes after VATS lung resection. Of course, there are other deciding factors such as no air leakage, but a threshold is mainly based on the experience of the surgeon’s predecessors and their own intuition more than evidence (23). This can lead to over-treatment which in turn induces other diseases such as lung infection. However, in this study, the experimental group had a satisfactory outcome with a lower rate of infection, compared with the control group.
Recently, according to several published studies, experts found no obvious correlation between drainage volume and the risk of complications, thus drainage volume is an independent factor and does not contribute to complications (9). However, several articles have also reported that the outcome after removal of the chest tube with high rates of effusion are not exciting, and the authors believed that if the chest tube was removed early this would lead to an increased rate of readmittance and a higher intervention rate (26). According to current research, no study has discussed the relationship between high-output drainage volume and the risk of complications. Therefore, in this study, drainage time became more prominent. As in previous research, drainage volume was the focus of the study. However, unfortunately, a consensus on when to remove the chest tube after VATS pulmonary resection has not yet been achieved. In this study, we changed the focus of our research, as we believed it is important to explore the relationship between drainage time and the risk of complications. We found that early removal of the chest tube led to a better short-term prognosis such as significantly shortened length of stay in hospital and low rates of infection.
The procedure of removing the chest tube early plays an important role at the stage of postoperative recovery after surgery, especially thoracic surgery. Also, patients in whom the chest tube is removed early have a potential decrease in the costs of hospitalization, since those patients have a shorter in-hospital stay (27). Therefore, in this study, the patients were divided into two groups in terms of drainage time; an experimental group and a control group. Regarding hospital stay, the experimental group was significantly shorter than the control group, and the rate of complications was also less than the control group. Comparing rates of lung infection and the necessity for reoperation, pleural effusion was not a very important factor. Lung infection could lead to many other problems such as increased costs and prolonged in-hospital stay, and it could induce other unexpected complications.
In addition, an article published by Lars stated that one could hypothesize that high drainage before the chest tube was removed caused accumulation of an amount of liquid in the pleural cavity, creating an ideal environment for bacterial breeding (7). This environment can easily lead to lung infections and chylothorax. Thus, it affects the patient’s postoperative recovery time. However, the results showing low rates of postoperative infection in this study contradict this hypothesis. Furthermore, some articles suggest that the absorption of pleural fluid is probably conducted through the lymphatic circulatory system rather than through the visceral pleura (28). Compared with the control group, the proportion of complications in the experimental group was lower. In addition, a chest tube may cause pain and affect the mobility of the chest, thus it has been suggested that removal of the chest tube improves lung function such as FEV1 (29), and may be beneficial to the recovery of vital capacity and physical fitness (30). The procedure of early removal of the chest tube after VATS should be encouraged based on this analysis which shows that it is safe and feasible. Early removal reduces the degree of pain, shortens hospital stay, improves lung function and reduces complications.
In conclusion, the procedure of early removal of the chest tube after VATS can result in a good short-term prognosis when the surgeon confirms that there is no air leakage, and the remaining lungs are completely expanded. Furthermore, early removal results in a shorter hospital stay and, importantly, decreases morbidity without the added risk of postoperative complications.
Limitation
Of course, in this study, some limitations were discovered. Firstly, this was a retrospective study and patients were collected based on historical controls. This could result in bias in the statistical results. Secondly, for chest tubes, according to the tumor position, surgeons could choose one or two tubes. The choice of one or two tubes may cause different influences on the experimental outcome. We should establish a unified standard during the experimental procedure. Third, this study is a single center experience-based analysis, thus we need to work together with more hospitals to obtain more evidence to prove that the result is reliable. Finally, because this is a retrospective study, of course, there will be some inevitable deviation in this study even though we tried to avoid it.
Acknowledgments
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Nomori H, Horio H, Fuyuno G, et al. Nonserratus-sparing antero-axillary thoracotomy with disconnection of anterior rib cartilage. Chest 1997;111:572-6. [Crossref] [PubMed]
- Nomori H, Horio H, Suemasu K. Anterior limited thoracotomy with intrathoracic illumination for lung cancer. Chest 1999;115:874-80. [Crossref] [PubMed]
- Shields TW, LoCicero III J, Ponn RB. General thoracic surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2000.
- Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin 2005;15:105-21. [Crossref] [PubMed]
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-84. [PubMed]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 mL/day. Eur J Cardiothorac Surg 2014;45:241-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802-A prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Nakanishi R, Fujino Y, Yamashita T, et al. A prospective study of the association between drainage volume within 24 hours after thoracoscopic lobectomy and postoperative morbidity. J Thorac Cardiovasc Surg 2009;137:1394-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. The distribution and likelihood of lymph node metastasis based on the lobar location of non-small cell lung cancer. Ann Thorac Surg 2006;81:1969-73. [Crossref] [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Trouble shooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52. [Crossref] [PubMed]
- McKenna R Jr. Vats lobectomy with mediastinal lymph node sampling or dissection. Chest Surg Clin N Am 1995;5:223-32. [PubMed]
- Zhang Ye, Li Hui, Hu Bin, et al. A prospective randomized single-blind control study of volume threshold for chest tube removal following lobectomy. World J Surg 2014;38:60-7. [Crossref] [PubMed]
- Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763-4. [Crossref] [PubMed]
- Kehlet H, Slim K. The future of fast-track surgery. Br J Surg 2012;99:1025-6. [Crossref] [PubMed]
- Hart R, Musfeldt C. MD-directed critical pathways: it's time. Hospitals 1992;66:56. [PubMed]
- Patton MD, Scaerf R. Thoracotomy critical pathways and clinical outcomes. Cancer Pract 1995;3:286-94. [PubMed]
- Cohen J, Stock M, Anderson P. Critical pathways for head and neck surgery: development and implementation. Arch Otolaryngol Head Neck Surg 1997;123:11-4. [Crossref] [PubMed]
- McKenna RJ Jr, Mahtabifard A, Pickens A, et al. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg 2007;84:1663-7; discussion 1667-8.
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Cerfolio RJ, Minnich DJ, Bryant AS. The removal of chest tubes despite an air leak or a pneumothorax. Ann Thorac Surg 2009;87:1690-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. The management of chest tubes after pulmonary resection. Thorac Surg Clin 2010;20:399-405. [Crossref] [PubMed]
- Göttgens KW, Siebenga J, Belgers EH, et al. Early removal of the chest tube after complete video-assisted thoracoscopic lobectomies. Eur J Cardiothorac Surg 2011;39:575-8. [Crossref] [PubMed]
- Grodzki T. Prospective algorithm to remove chest tubes after pulmonary resection with high output-is it valid everywhere? J Thorac Cardiovasc Surg 2008;136:536-7. [Crossref] [PubMed]
- Bardell T, Petsikas D. What keeps postpulmonary resection patients in hospital? Can Respir J 2003;10:86-9. [Crossref] [PubMed]
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219-25. [Crossref] [PubMed]
- Refai M, Brunelli A, Salati M, et al. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg 2012;41:820-2. [Crossref] [PubMed]
- Nomori H, Horio H, Suemasu K. Early removal of chest drainage tubes and oxygen support after a lobectomy for lung cancer facilitates earlier recovery of the 6-minute walking distance. Surg Today 2001;31:395-9. [Crossref] [PubMed]


