
The combination of preoperative D-dimer and CA19-9 predicts lymph node metastasis and survival in intrahepatic cholangiocarcinoma patients after curative resection
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignant tumor in the liver, which is originated from hepatobiliary tract and have worse prognosis than hepatocellular carcinoma (1). Surgical resection, hepatic resection and lymph node dissection, is the only potentially curative treatment for ICC patients (2,3). However, a 5-year survival rate after resection merely increased 15–40% and up to 50–70% of patients still have tumour recurrence after radical resection (1,4). Different from hepatocellular carcinoma, lymph node metastasis (LNM) is common in ICC, which is reported to be one of the most important poor prognostic factors for ICC patients following curative resection (5,6). The ability to preoperatively predict lymph node status would aid clinical decisions regarding therapeutic strategies for ICC patients. Therefore, useful preoperative biomarkers for prediction of LNM and to predict the prognosis of ICC patients after resection are urgently needed.
D-dimer is an important marker in clinic for thromboembolism, such as deep venous thromboembolism (DVT), and recently it has been found to be associated with progression and recurrence of several malignant diseases including colorectal liver metastasis (7-9). However, no studies have investigated the relationship between serum D-dimer levels and LNM and the prognosis of ICC. Carbohydrate antigen 19-9 (CA19-9) is frequently used as a tumour biomarker for detection and prediction of adenocarcinomas, while it was also reported as an independent prognostic factor of ICC (10,11). Therefore, we established a new parameter, the combination of preoperative D-dimer and CA19-9 (CPDC), and hypothesized that CPDC might have better prediction value for LNM and prognosis in ICC patients after curative liver resection, which might be helpful to make surgical strategies and follow-up plans.
Methods
Patients
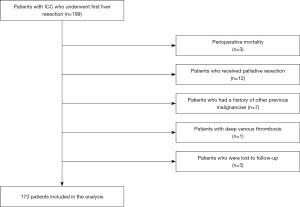
Clinical and pathological data were collected retrospectively from 173 patients admitted to our hospital between April 2012 and December 2018. The eligibility criteria were as follows: (I) complete resection of liver tumours with a gross-negative surgical margin and histopathological diagnosis of ICC; (II) no history of other previous malignancies. The exclusion criteria included: (I) the presence of clinical or pathological distant metastases; (II) perioperative mortality; (III) loss of follow-up data and (IV) patients with preoperatively acute and chronic infection [joint infection, chronic obstructive pulmonary disease (COPD) et al.], inflammation, HIV-infected and diseases of blood system associated with D-dimer levels (deep venous thrombosis, pulmonary embolism or disseminated intravascular coagulation et al.). Flow diagram for the selection of ICC patients included in the final analyses was showed in Figure 1. The Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences approved the study (ID: LC2018A15), and the necessity for informed consent was waived.
Hematologic examinations including the serum tumour markers carcinoembryonic antigen (CEA) and CA19-9, routine blood tests and liver function tests, were performed within 1 week before operation. An electrochemiluminescence immunoassay (ECLI) method was established to detect serum CA19-9 and CEA. An enzyme-linked fluorescent immunoassay was used to evaluate serum D-dimer concentrations. We mainly take the following measures to ensure the accuracy of the laboratory detection: (I) in clinical laboratory detection, the examiners carry on the check and maintenance to the detection instruments every day to ensure the normal operation of the detection instruments; (II) every sample is tested for at least three times and the results are averaged; (III) after clinical laboratory detection, the examiners shall repeatedly check the test results by two persons and record the test results in detail. In our hospital, the normal reference range of the serum D-dimer levels was 0 to 0.55 mg/L, and the normal reference range of the CA19-9 levels was between 0 and 27 U/mL. The combination of the preoperative D-dimer with CA19-9 levels (CPDC) was established to analyse the prognostic value of LNM and survival. The CPDC was scored as 0 (decreased D-dimer levels with decreased CA19-9 levels), 2 (elevated D-dimer levels with elevated CA19-9 levels), or 1 (all other combinations).
Treatment and follow-up
Decisions about the treatment strategy have been described previously (12). Treatment-related factors included preoperative chemotherapy and radiotherapy, extent of hepatic resection, lymph node dissection, duration of inpatient hospital stay, postoperative complications, and postoperative chemotherapy and radiotherapy. Chemotherapy, radiation therapy, transhepatic arterial chemotherapy and embolization (TACE), percutaneous ablation and repeated surgery were applied for recurrent patients. All postoperative complications were graded according to the Clavien-Dindo classification system (13). Progression-free survival (PFS) and overall survival (OS) were used to estimate the survival of ICC patients after resection. The first endpoint of survival was the date of recurrence and the second endpoint of survival was the date of death. PFS was calculated from the date of the operation to the date of recurrence or the last follow-up time. OS was defined as the length of time from the date of hepatic resection to the last follow-up or death. Patients underwent first postoperative CT or MRI examination and hematological examination 1 month after surgery. After this, they were required to visit the clinics every 3 months during the first 2 postoperative years, every 6 months thereafter for 3 years, and yearly after 5 years. This study followed up the patients by telephone and searching for patient check data in hospital medical record system after each check. The deadline of follow-up was the date of death or the last follow-up date. The last follow-up date of this study was May 2019. During the follow-up, three patients were lost to follow-up.
Statistical analysis
The frequency distribution of categorical variables was compared with the chi-square test or Fisher’s exact test when appropriate and that of continuous variables was compared with the Mann-Whitney U test. A receiver operating characteristic curve (ROC) analysis was used to select the best cut-off value and the highest Youden index was used to investigate the optimal cut-off value. According to the optimal cut-off point, the pre-operative D-dimer levels and CA19-9 levels were classified into the high and low subgroups. The area under the ROC curve was used to evaluate the prediction accuracy. Clinicopathological factors with a P<0.1 for significance in univariate analysis were included in multivariate analysis. A multivariate logistic regression analysis for LNM was performed to identify independent factors. The OS and PFS were compared using the Kaplan-Meier method; comparisons were performed with the log-rank test. Multivariate analyses of survival were conducted by cox regression models. Forward: likelihood ratio (LR) was used in multivariate analysis. P<0.05 was considered statistically significant. SPSS, version 21 software (Armonk, NV, USA) was used to perform the statistical analyses.
Results
Clinicopathological characteristics
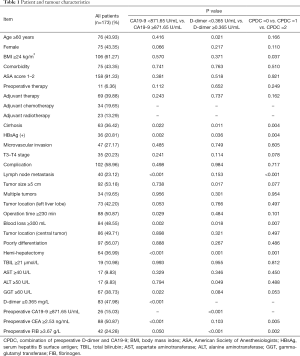
Among all 173 patients, 75 patients (43.35%) were female, and the median age [interquartile range (IQR)] was 58.00 (51.50–64.00) years. Thirty-six patients (20.81%) had positive serum hepatitis B surface antigen (HBsAg), and 63 patients (36.42%) had cirrhosis. Preoperative therapy was administered to 11 patients (6.36%), and postoperative therapy was administered to 69 patients (39.88%) (adjuvant chemotherapy: 34 cases; adjuvant radiotherapy: 23 cases; adjuvant chemotherapy combined with adjuvant radiotherapy: 9 cases; other: 3 cases). Seventy-three patients (42.20%) had lesions in left liver lobe. The median diameter of the largest lesion was 5.10 (IQR, 3.70–7.00) cm, and 53.18% of patients had a lesion larger than 5 cm. Nineteen-point-six-five percent of patients had multiple tumours and 56.07% of patients had tumours in poor differentiation. Twenty-three-point-one-two percent of the patients had LNM. Sixty-four patients (36.99%) underwent hemi-hepatectomy. In this study, 58.96% (102/173) of the patients experienced postoperative complications, including 37 major complications (37/102, 36.27%) and 65 minor complications (65/102, 63.73%). The clinicopathological characteristics of the patients are listed in Table 1.
Full table
Prognostic value of D-dimer levels and CA19-9 levels for survival
The median follow-up period was 35.00 months. A total of 112 patients (64.74%) experienced recurrence, and 79 patients (45.66%) died. The median OS was 25.00 months [95% confidence interval (CI): 9.96–40.04], and the median PFS was 10.00 months (95% CI: 7.21–12.79). The 1-, 3- and 5-year survival rates were 70.99%, 45.51% and 38.58%, respectively. The 1-, 3- and 5-year PFS rates were 41.45%, 29.46%, and 21.46%, respectively.
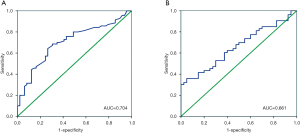
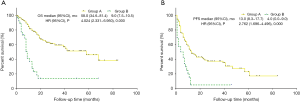
The median survival time (25.00 months) was considered an endpoint. The best cut-off value of the preoperative D-dimer levels and CA19-9 levels for predicting survival was determined by the ROC curves (Figure 2). The optimal cut-off level for D-dimer was 0.365 mg/L, and the area under the curve (AUC) was 0.704 (95% CI: 0.610–0.798, P<0.001). This value was associated with a sensitivity of 0.686 and a specificity of 0.694. Ninety patients (52.02%) had a D-dimer <0.365 mg/L. For CA19-9 levels, the optimal cut-off level was 871.65 U/mL, and the AUC was 0.661 (95% CI: 0.552–0.769, P=0.007). This value was associated with a sensitivity of 0.358 and a specificity of 0.950. Twenty-six patients (15.03%) had a CA19-9 ≥871.65 U/mL.
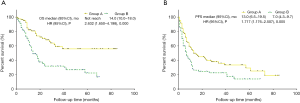
Patients with a D-dimer level ≥0.365 mg/L had significantly worse OS and PFS than patients with a D-dimer level <0.365 mg/L (P<0.001, median OS: 14.00 months versus not reached; P=0.005, median PFS: 7.00 versus 13.00 months) (Figure 3). Patients with a CA19-9 level ≥871.65 U/mL had a significantly worse OS and PFS than patients with a CA19-9 level <871.65 U/mL (P<0.001, median OS: 9.00 versus 58.00 months; P<0.001, median PFS: 4.00 versus 13.00 months) (Figure 4).
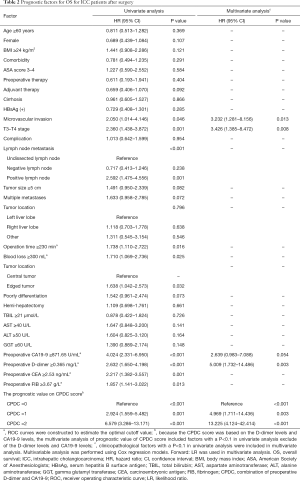
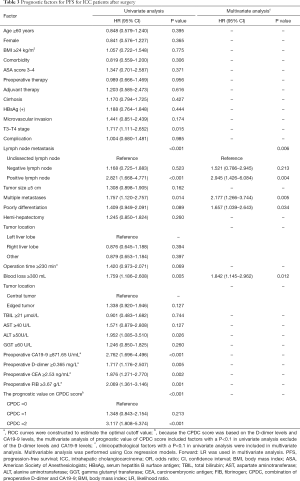
The univariate analysis revealed D-dimer levels ≥0.365 mg/L (P<0.001), CA19-9 ≥871.65 U/mL (P<0.001), microvascular invasion (P=0.046), T3–T4 stage (P=0.001), LNM (P<0.001), operation time ≥230 min (P=0.016), blood loss ≥300 mL (P=0.025), tumour location (central tumour) (P=0.032), preoperative CEA ≥2.53 ng/mL (P=0.001) and preoperative fibrinogen (FIB) ≥3.67 g/L (P=0.013) were all associated with shorter OS (Table 2). These factors were included in the multivariate analysis. In the multivariate analysis, elevated preoperative D-dimer levels [hazard ratio (HR) =5.009, 95% CI: 1.732–14.486, P=0.003), T3–T4 stage (HR =3.426, 95% CI: 1.385–8.472, P=0.008) and microvascular invasion (HR =3.232, 95% CI: 1.281–8.156, P=0.013) remained independently associated with shorter OS (Table 2). In the univariate analysis of the PFS, D-dimer levels ≥0.365 mg/L (P=0.005), CA19-9 ≥871.65 U/mL (P<0.001), T3–T4 stage (P=0.015), LNM (P<0.001), multiple metastases (P=0.014) and preoperative CEA ≥2.53 ng/mL (P=0.002) significantly decreased PFS. These factors were included in the multivariate analysis. In the multivariate analysis, the elevated preoperative D-dimer levels and elevated preoperative CA19-9 levels were not independent factors associated with this reduction in PFS (Table 3).
Full table
Full table
Prognostic value of CPDC for survival
The CPDC score had an AUC of 0.756 (95% CI: 0.658–0.854, P<0.001) with a sensitivity of 0.778 and a specificity of 0.659. For predicting survival, the AUC for the CPDC score was stronger than the D-dimer levels (P=0.009, AUC: 0.756 versus 0.707) and CA19-9 levels (P=0.010, AUC: 0.756 versus 0.661). Because the CPDC score was based on the D-dimer levels and CA19-9 levels, the multivariate analysis of prognostic value of CPDC score included factors with a P<0.1 in the univariate analysis exclude of the D-dimer levels and CA19-9 levels. In the multivariate analysis, the CPDC score was an independent prognostic factor for OS and was not an independent factor for PFS (Tables 2,3).
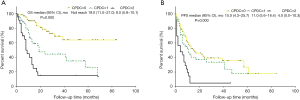
Kaplan-Meier curve analysis showed that compared with a CPDC =1 or CPDC =0, CPDC =2 was significantly associated with worse OS (P<0.001, median OS: 8.00 versus 19.00 months versus not reached, respectively) and worse PFS (P<0.001, median PFS: 4.00 versus 11.00 versus 15.00 months, respectively) in patients (Figure 5). The differences between any two groups were significant for the OS comparisons (CPDC =0 versus CPDC =1, P<0.001; CPDC =0 versus CPDC =2, P<0.001; CPDC =1 versus CPDC =2, P=0.008).
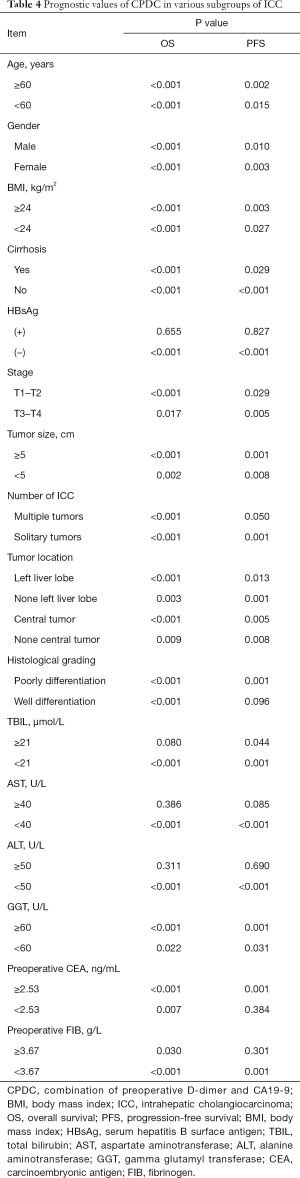
Prognostic value of CPDC in various subgroups of ICC
Prognostic significance of preoperative CPDC was evaluated in various subgroups of ICC patients then (Table 4). As seen in the Table 4, the predictive value of increased preoperative CPDC for a poorer OS or PFS in almost all subgroups except the HBsAg (+) group, aspartate aminotransferase (AST) ≥40 U/L group and alanine aminotransferase (ALT) ≥50 U/L group.
Full table
Predictors for LNM
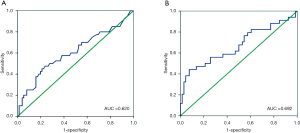
ROC curves were generated to illustrate the ability of preoperative D-dimer and CA19-9 levels to predict LNM. For D-dimer, the optimal cut-off level was 0.575 mg/L, and the AUC was 0.620 (95% CI: 0.509–0.730, P=0.034) with a sensitivity of 0.450 and a specificity of 0.789 (Figure 6). Eighty-two patients had a D-dimer level <0.575 mg/L. For CA19-9, the optimal cut-off level was 997.80 U/mL, and the AUC was 0.692 (95% CI: 0.576–0.808, P=0.001) with a sensitivity of 0.441 and a specificity of 0.924. Eighty-three patients had a CA19-9 level <997.80 U/mL.
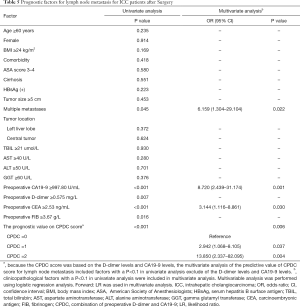
The predictors for LNM were analysed. In the univariate analysis, multiple metastases (P=0.045), preoperative CA19-9 ≥997.80 (P<0.001), preoperative D-dimer ≥0.575 (P=0.007), preoperative CEA ≥2.53 ng/mL (P<0.001) and preoperative FIB ≥3.67 (P=0.016) were statistically significant with LNM. These statistically significant parameters in the univariate analysis were retained in the multivariate model. In the multivariate analysis, elevated preoperative CA19-9 levels [odds ratio (OR) =8.720, 95% CI: 2.439–31.174, P=0.001], multiple metastases (OR =6.159, 95% CI: 1.304–29.104, P=0.022) and elevated preoperative CEA levels (OR =3.144, 95% CI: 1.116–8.861, P=0.030) significantly predicted LNM (Table 5).
Full table
Because the CPDC score was based on the D-dimer levels and CA19-9 levels, the multivariate analysis of the predictive value of CPDC score for lymph node metastasis included factors with a P<0.1 in univariate analysis exclude of the D-dimer levels and CA19-9 levels. In the multivariate logistic regression analysis, the CPDC score independently predicted LNM (Table 5). The AUC for the CPDC score was 0.722 (95% CI: 0.613–0.831, P<0.001). This value was associated with a sensitivity of 0.657 and a specificity of 0.739.
Relationships among D-dimer, CA19-9, CPDC and clinicopathological characteristics
The clinicopathologic factors of patients with different D-dimer, CA19-9 and CPDC statuses are summarized in Table 1. Age ≥60 years (P=0.021), cirrhosis (P=0.011), HBsAg (+) (P=0.036) and tumour size ≥5 cm (P=0.017) were observed in the elevated D-dimer level group. Elevated CA19-9 levels were significantly associated with cirrhosis (P=0.022) and HBsAg positivity (P=0.002). The increased CPDC was associated with body mass index (BMI) ≥24 kg/m2 (P=0.037), cirrhosis (P=0.004) and HBsAg positivity (P=0.004).
Discussion
Among current researches about ICC, this study seems to be the first to describe the relationships between preoperative D-dimer levels, CPDC and survival and LNM in ICC patients. We found that increased preoperative D-dimer levels, CA19-9 levels and CPDC were associated with worse survival and LNM in ICC patients after hepatic resection, and CPDC score had stronger predictive ability than that of D-dimer or CA19-9 levels alone, indicating that CPDC could be used as a useful prognostic marker for ICC patients in clinics preoperatively.
This study revealed that a poor prognosis of ICC patients was associated with high D-dimer levels. Furthermore, elevated D-dimer levels were correlated with worse prognosis in lung cancer, ovarian cancer, colorectal liver metastases and renal cancer patients in several studies (7-9,14). These findings are consistent with the results of the current study. The reasons of the association between elevated D-dimer levels and poor prognoses are as follows: malignant tumour cells can express a large amount of procoagulant molecules and promote the coagulation system and fibrinolysis (15,16). Serum D-dimer levels, a sign of abnormal fibrinolysis, increase with fibrin degradation. D-dimer promotes proliferation and induces angiogenesis by modulating cellular signalling (17). Moreover, some researches have revealed that serum D-dimer levels are positively related to the presence of circulating tumour cells (CTCs) or micro-metastases, which are associated with poor survival (18,19). Our study also indicated that increased serum CA19-9 levels were an independent prognostic factor for poor prognosis. This finding parallel those of previous studies indicating that serum CA19-9 is significantly correlated with inferior survival in ICC patients who underwent surgery (10,11). The preoperative serum CA19-9 level was identified as having a significant relationship with the CTC count in cholangiocarcinoma, and the CTC count was associated with a significantly shorter survival (20).
To increase the predictive value, we combined the preoperative D-dimer with CA19-9 to establish a new parameter, CPDC. Our results suggest that the predictive capacity of the CPDC score was stronger than any one parameter alone. Moreover, patients with a CPDC score =2 had a poorer OS and PFS than patients with a CPDC score =1 or CPDC score =0. Further, there were significant differences between any two groups in the OS comparisons. Particularly in the subgroup analysis, the predictive value of an increased preoperative CPDC for worsened OS and PFS existed in almost all subgroups. These results indicate that preoperative CPDC was an accurate marker to predict the survival of ICC patients after resection, especially in various subgroups of ICC patients whose prognoses are difficult to estimate.
LNM is an important prognostic factor for ICC patients following resection (5,6). The results of this study suggested that the preoperative level of D-dimer and CA19-9 have potential as predictors of LNM in ICC patients. Some studies have revealed that serum D-dimer levels are a potential marker for predicting distant metastasis in various cancer types (21,22). Our study first found a relationship between D-dimer levels and LNM in ICC patients. The underlying mechanism may be the following: Metastasis signifies the escape of tumour cells from the primary tumour site their entry into the peripheral blood or lymph system (tumours in the lymph system can enter peripheral blood through lymphatic circulation). During migration, micrometastatic tumour cells express tissue factors to activate coagulation and fibrinolysis (23,24). Serum D-dimer levels are widely used biomarkers for the activation of coagulation and fibrinolysis. In addition, elevated D-dimer levels can induce the growth and spread of tumour cells through many mechanisms (24-26). Several reports have indicated that elevated CA19-9 levels are associated with a high ratio of LNM (11,27), which suggests that high CA19-9 levels could signify the presence of metastatic tumour cells (11,20). Interestingly, the results of our study indicated that CPDC had a strong predictive value for LNM with high sensitivity and specificity and was an independent predictive factor for LNM. Early, accurate detection of LNM is important for patient prognosis and treatment (either lymph node resection or the timing of chemotherapy and radiotherapy). Imaging techniques such as CT and MRI are commonly used for diagnosing LNM. By comparison, blood tests are relatively non-invasive, rapid, economical, and repeatable, and ionizing radiation is not used. Blood tests for D-dimer and CA19-9 could become common indicators to determine whether further detailed imaging examination is warranted. In addition, serum biomarkers are more suitable for long-term follow-up. In the future, we will investigate the predictive value of imaging techniques combined with this indicator for LNM.
The CPDC score is not only accurate but also clinically meaningful. Prior to resection, the predictive value of the CPDC score can help clinicians identify patients at high risk for LNM and a poor prognosis and guide decisions regarding individual treatment strategies according to the patient’s CPDC score. ICC patients with a CPDC score =2 were more prone to LNM and a poor prognosis after liver resection, and these patients should be provided with closer surveillance and even targeted adjuvant therapy.
According to previous studies, elevated D-dimer levels were significantly associated with larger tumours, multiple metastases and elevated CEA levels in several malignancies (7,28). Elevated CA19-9 levels were demonstrated to be significantly correlated with a high Child-Pugh score, large tumour size, and high TNM stage for ICC patients (10). Notably, our study found that elevated D-dimer levels and elevated CA19-9 levels were correlated with cirrhosis, HBsAg positivity, and LNM in ICC patients.
It is worth noting that the results of our study showed that LNM was an independent prognostic factor of PFS but not for OS. However, some studies have shown that LNM is predictive for the PFS and OS of ICC patients (10,29). The reason may be that the samples in this study included both patients with and those without lymph node resection, which were divided into three categories: no lymph node resection, positive LNM and negative LNM. Whether positive LNM is an independent risk factor for prognosis under this classification is not very clear. The samples in other studies included only patients with lymph node resection, which were divided into two categories: positive LNM and negative LNM. The difference between the samples and the classification variables leads to differences in the results. In addition, multivariate analysis in this study showed that positive LNM was an independent prognostic factor in patients with lymph node resection, which was consistent with other studies (10,29). For ICC patients, lymph node resection is recommended (30-32), which was always followed in this study. However, the diagnostic criteria of preoperative ICC depend on the imaging characteristics and tumour markers of the patients. In this study, some patients did not receive lymph node resection because the preoperative clinical features were not typical.
The present study does have some limitations. First, this was a single-centre retrospective study and the sample size was relatively small. Small sample size might affect the identified power. In addition, some biases exist, such as a lack of random assignment and patient selection bias. Second, there was no validation setting to validate the results because of the small sample size. In the future, we will further expand the sample size to construct a validation setting and verify our results. We look forward to conducting prospective cohort studies to improve the limitations in this study.
In conclusion, our study revealed that preoperative CPDC is a novel independent factor for poor prognosis and LNM in ICC patients. The measurement of CPDC is based on standard laboratory measurements of D-dimer and serum tumour marker CA19-9, which is in clinical routine. Therefore, CPDC is a convenient and meaningful prognostic biomarker for ICC prognosis, which has reference significance for operation strategies about lymph node dissection and early therapeutic intervention according to different clinicopathological characteristics.
Acknowledgments
Funding: This work was supported by the State Key Project on Infection Diseases of China (Grant No. 2017ZX10201021-007-003), the National Capital Health Research and Development of Special (No. 2018-1-4021), the Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2018A15) and the National Natural Science Foundation of China (No. 81672461).
Footnote
Conflicts of Interest: HZ serves as an unpaid Associate Editors-in-Chief of Annals of Translational Medicine from Jun 2019 to May 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was in compliance with the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences (ID: LC2018A15), and the necessity for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Yoh T, Hatano E, Nishio T, et al. Significant Improvement in Outcomes of Patients with Intrahepatic Cholangiocarcinoma after Surgery. World J Surg 2016;40:2229-36. [Crossref] [PubMed]
- Ruzzenente A, Conci S, Valdegamberi A, et al. Role of surgery in the treatment of intrahepatic cholangiocarcinoma. Eur Rev Med Pharmacol Sci 2015;19:2892-900. [PubMed]
- Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg 2018;105:848-56. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Chen Q, Zhao H, Wu J, et al. Preoperative D-dimer and Gamma-Glutamyltranspeptidase Predict Major Complications and Survival in Colorectal Liver Metastases Patients After Resection. Transl Oncol 2019;12:996-1004. [Crossref] [PubMed]
- İnal T, Anar C, Polat G, et al. The prognostic value of D-dimer in lung cancer. Clin Respir J 2015;9:305-13. [Crossref] [PubMed]
- Tas F, Kilic L, Bilgin E, et al. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer 2013;23:276-81. [Crossref] [PubMed]
- Zhang Y, Shi SM, Yang H, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer 2019;10:494-503. [Crossref] [PubMed]
- Yamada T, Nakanishi Y, Okamura K, et al. Impact of serum carbohydrate antigen 19-9 level on prognosis and prediction of lymph node metastasis in patients with intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Chen Q, Wu C, Zhao H, et al. Neo-adjuvant Chemotherapy-Induced Neutropenia Is Associated with Histological Responses and Outcomes after the Resection of Colorectal Liver Metastases. J Gastrointest Surg 2020;24:659-70. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Erdem S, Amasyali AS, Aytac O, et al. Increased preoperative levels of plasma fibrinogen and D dimer in patients with renal cell carcinoma is associated with poor survival and adverse tumor characteristics. Urol Oncol 2014;32:1031-40. [Crossref] [PubMed]
- Kwaan HC, Lindholm PF. Fibrin and Fibrinolysis in Cancer. Semin Thromb Hemost 2019;45:413-22. [Crossref] [PubMed]
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013;11:223-33. [Crossref] [PubMed]
- McCluney SJ, Giakoustidis A, Segler A, et al. Neutrophil: Lymphocyte ratio as a method of predicting complications following hepatic resection for colorectal liver metastasis. J Surg Oncol 2018;117:1058-65. [Crossref] [PubMed]
- Mego M, Zuo Z, Gao H, et al. Circulating tumour cells are linked to plasma D-dimer levels in patients with metastatic breast cancer. Thromb Haemost 2015;113:593-8. [Crossref] [PubMed]
- Thaler J, Ay C, Mackman N, et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost 2012;10:1363-70. [Crossref] [PubMed]
- Yang JD, Campion MB, Liu MC, et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology 2016;63:148-58. [Crossref] [PubMed]
- Rong G, Zhang M, Xia W, et al. Plasma CADM1 promoter hypermethylation and D-dimer as novel metastasis predictors of cervical cancer. J Obstet Gynaecol Res 2019;45:1251-9. [Crossref] [PubMed]
- Dai H, Zhou H, Sun Y, et al. D-dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed Rep 2018;9:453-7. [PubMed]
- Guo Y, Chen F, Cui W. Usefulness of plasma D-dimer level for monitoring development of distant organ metastasis in colorectal cancer patients after curative resection. Cancer Manag Res 2018;10:4203-16. Erratum in: Erratum: Usefulness of plasma D-dimer level for monitoring development of distant organ metastasis in colorectal cancer patients after curative resection [Corrigendum]. [Cancer Manag Res. 2018]. [Crossref] [PubMed]
- Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 2005;105:1734-41. [Crossref] [PubMed]
- Diao D, Cheng Y, Song Y, et al. D-dimer is an essential accompaniment of circulating tumor cells in gastric cancer. BMC Cancer 2017;17:56. [Crossref] [PubMed]
- Buller HR, van Doormaal FF, van Sluis GL, et al. Cancer and thrombosis: from molecular mechanisms to clinical presentations. J Thromb Haemost 2007;5 Suppl 1:246-54. [Crossref] [PubMed]
- Meng ZW, Lin XQ, Zhu JH, et al. A nomogram to predict lymph node metastasis before resection in intrahepatic cholangiocarcinoma. J Surg Res 2018;226:56-63. [Crossref] [PubMed]
- Jiang X, Mei X, Wu H, et al. D-dimer level is related to the prognosis of patients with small cell lung cancer. Ann Transl Med 2017;5:394. [Crossref] [PubMed]
- Li T, Qin LX, Zhou J, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int 2014;34:953-60. [Crossref] [PubMed]
- Bektas H, Yeyrek C, Kleine M, et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci 2015;22:131-7. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013;17:1917-28. [Crossref] [PubMed]
- Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [Crossref] [PubMed]


