Transanal versus nontransanal surgery for the treatment of primary rectal gastrointestinal stromal tumors: a 10-year experience in a high-volume center
Introduction
The gastrointestinal stromal tumor (GIST) is a tumor type with a malignant tendency originating from mesenchymal tissue. The incidence rate is 1–2/100,000, accounting for approximately 20% of all soft tissue sarcomas (1-3). Such tumors can occur throughout the digestive tract, with the stomach being the most common site, accounting for approximately 60%, while the rectum is relatively rare, accounting for approximately 5% (4,5). Because of the low incidence and lack of evidence from large-sample, prospective studies, predictive behavioral data in the National Comprehensive Cancer Network (NCCN) guidelines for rectal GIST are mainly derived from a retrospective study of 111 cases in 2006 (6,7). At present, the diagnosis and treatment of rectal GIST still refers to the guidelines of gastric GIST and a modified National Institutes of Health (NIH) risk grading system (index contains tumor site, tumor size, mitotic count and rupture), including very low-risk, low-risk, intermediate-risk and high-risk, was used to predict recurrence risk (8). In recent years, it is clear that this type of disease has a malignant tendency and is prone to recurrence, and the prognosis is worse than that of gastric GIST (9,10). At present, preoperative treatment, surgical approach, resection scope and prognosis of rectal GIST are still inconclusive, with much controversy (4,11). This study retrospectively collected 64 surgically resected rectal GISTs admitted to the Sixth Affiliated Hospital of Sun Yat-sen University from 1998 to 2018. All data were divided into two groups: the transanal (TA) group and the nontransanal (NTA) group. Then, clinicopathology and prognosis were compared in these two groups.
Methods
We received ethical approval for this case series from the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China and obtained consent for publication from the patients.
Patients
A retrospective collection of rectal GIST cases was performed at the Sixth Affiliated Hospital of Sun Yat-sen University from 2008 to 2018. Enrollment criteria: (I) complete clinical information and follow-up and (II) primary GIST and pathological diagnosis. Exclusion criteria: (I) combined with other malignant tumors, (II) multiple GIST and (III) deaths due to other diseases.
Observation indicators and follow-up
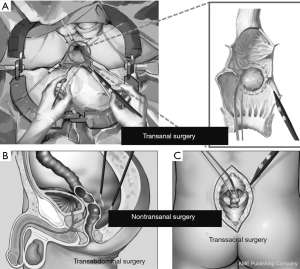
For the surgical approach, the enrolled cases were divided into the TA group and the NTA group. According to previous surgical records, TA surgery was defined as the application of lithotomy or folding position, and local resection was performed under direct vision or utilizing a transanal endoscopy microsurgery (TEM) platform. NTA surgery was defined as trans-sacral or transabdominal partial resection or radical surgery (Dixon, Miles surgery) (Figure 1). The clinical and pathological parameters, including age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, genetic test, preoperative treatment, surgical and postoperative outcomes, and pathological outcomes were retrospectively analyzed. According to the modified NIH risk grading system in 2008 (12), the disease is classified into very low-risk, low-risk, intermediate-risk and high-risk. The mitotic index was counted per 50/high power field (HPF), and the above pathological examinations were approved by three experienced pathologists. The start time of the study was defined as the surgical time, and the last follow-up time was 2019-02-15.
Statistics
Statistical analyses were performed using SPSS 19.0. Quantitative data are reported as the mean ± standard deviation (SD) or median. Categorical data were compared by χ2 tests or Fisher’s exact test. Survival curves [overall survival (OS) and disease-free survival (DFS)] were derived from Kaplan-Meier estimates, and the curves were compared by the log-rank test. A P value <0.05 was considered statistically significant.
Results
Patients
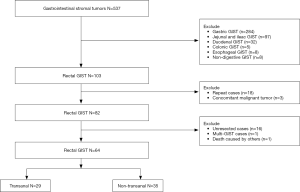
A total of 537 GIST cases were collected from the Sixth Affiliated Hospital of Sun Yat-sen University from 2008 to 2018. A total of 82 patients with rectal GIST who met the criteria for inclusion in this study accounted for 15.3% (82/537) of the total. There were 64 surgical resection cases, 29 cases in the TA group, and 35 cases in the NTA group (Figure 2).
Clinical information
The median age was 59 years old in the whole group. For sex, the male: female ratio was 41:23. The mean BMI was 22.3±2.4 kg/m2. For the ASA score, grade I: 5 cases (7.8%), grade II: 45 cases (70.3%), and grade III: 14 cases (21.9%). There were 41 cases with clinical symptoms in the whole group, including bloody stool (14 cases), anal pain (13 cases), abdominal pain (8 cases), difficulty in defecation (3 cases), change of bowel habits (2 cases), and frequent urination (1 case). In the diagnostic workup, 24 cases (37.5%) were diagnosed by needle biopsy, 38 cases (59.4%) were diagnosed by resection, and 2 cases (3.1%) were diagnosed by endoscopy. There were 30 cases (46.9%) with postoperative adjuvant therapy in the whole group. Ten cases (15.9%) underwent genetic tests. Twelve cases had a recurrence. There was no significant difference in age (P=1.000), gender (P=0.443), BMI (P=0.171), presenting symptoms (P=0.667), diagnostic workup (P=0.457), genetic test (P=0.490) and recurrence (P=1.000) between the TA and NTA groups. However, for the preoperative adjuvant treatment, 7 cases were in the TA group and 22 cases were in the NTA group, and there was a significant difference between the two groups (P=0.003). For the postoperative adjuvant treatment, 9 cases were in the TA group and 21 cases were in the NTA group, and there was a significant difference between the two groups (P=0.017) (Table 1).
Full table
Surgical and postoperative outcomes
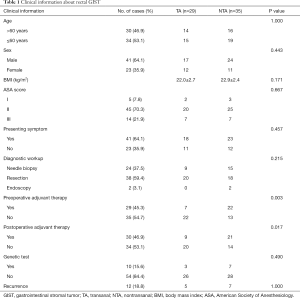
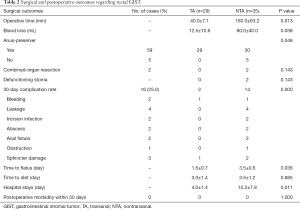
In terms of surgical index, the TA group had less operative time (40.0±7.1 vs. 160.0±63.2 min, P=0.013), less blood loss (12.5±10.6 vs. 80.0±40.0 mL, P=0.038), and a high anus-preserver rate (P=0.048). In the NTA group, there were 2 cases with combined-organ resection, one with combined-urinary bladder resection and one with combined-ovary resection. The overall postoperative 30-day complication rate was 25.0% (16/64), including 2 cases of hemorrhage, 4 cases of anastomotic leakage, 2 cases of incision infection, 2 cases of abscess, 2 cases of anal fistula, 1 case of small intestinal obstruction, and 1 case of sphincter damage. The 30-day postoperative complication rate was lower in the TA group than in the NTA group (P=0.000). In terms of the postoperative recovery index, the TA group had an earlier flatus time (1.5±0.7 vs. 3.5±0.6 days, P=0.036) and a shorter hospital stay (4.0±1.4 vs. 10.2±7.9 days, P=0.011). There was no postoperative morbidity within 30 days in the two groups (Table 2).
Full table
Pathological outcomes
In terms of tumor size, there were 8 cases (12.5%) ≤2 cm, 38 cases (59.4%) >2 & ≤5 cm, and 18 cases (28.1%) >5 & ≤10 cm. The tumors in the TA group were smaller than those in the NTA group, and there was a significant difference between the two groups (P=0.002). The distance from the anus in the TA group was shorter than that in the NTA group (4.2±0.9 vs. 5.8±2.1 cm, P=0.047). For tumor location, 26 cases (40.6%) were located in the anterior wall, 5 cases (7.8%) were located in the posterior wall, and 33 cases (51.6%) were located in the sidewall. In histopathological classification, there were 56 cases (87.5%) of spindle cell type, 7 cases (10.9%) of epithelial cell type, and 1 case (1.6%) of mixed type. Immunohistochemistry (IHC) was performed in all cases, including 52 cases of CD34(+), 60 cases of CD117(+), and 52 cases of Dog-1(+). There were only 10 cases with genetic mutation detection in the whole group, including 9 cases of c-Kit 11 mutation and 1 case of platelet-derived growth factor receptor alpha (PDGFRA) 12 mutation. Only one patient in the whole group had intraoperative tumor rupture. Only one case had a positive surgical margin and this case was treated by Imatinib later, now still be alive. There was no significant difference in tumor position (P=0.234), histopathological classification (P=0.623), IHC (P=0.442), genetic mutation test (P=0.347), tumor rupture (P=0.997) and surgical margin (P=0.997) between the TA group and the NTA group. However, for the mitotic count (P=0.035) and NIH criteria (P=0.000), there was a statistically significant difference between the TA group and the NTA group (Table 3).
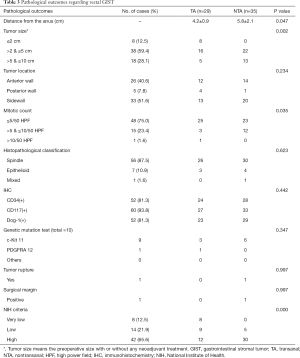
Full table
Some clinical information in different periods
The time between 2008 and 2018 was divided into four periods: 2008–2009, 2010–2012, 2013–2015, and 2016–2018. From Table 4, the resected cases gradually increased, which was 2 cases, 15 cases, 16 cases, and 31 cases, respectively, in the four different periods. The cases (%) that underwent trans-sacral surgery were 0 (0%), 1 (6.7%), 7 (43.8%) and 9 (29.0%), respectively, while the cases (%) of extensive resection were 0 (0%), 4 (26.7%), 5 (31.3%) and 6 (19.4%), respectively. In the whole group, only 2 cases (12.5%) were in 2013–2015, and 8 cases (25.8%) in 2016–2018 performed a genetic test. The cases (%) of neoadjuvant therapy in the four-time periods were 0 (0%), 4 (26.7%), 9 (56.3%), and 16 (51.6%), respectively, and the cases (%) of postoperative adjuvant treatments were 1 (50.0%), 1 (6.7%), 8 (50.0%), and 20 (64.5%), respectively.
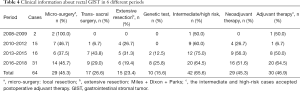
Full table
Prognosis
The mean overall follow-up time was 46 (range, 1–122) months. Disease recurrence over the entire follow-up period was observed in 17.2% (n=5) of patients in the TA group and 20.0% (n=7) of patients in the NTA group, without a significant difference between these two groups (P=1.000) (Table 1). Among the 12 recurrence cases, 10 cases were recurrent in situ, 1 case had liver metastasis and recurrence in situ, and 1 case had prostate and seminal vesicle metastasis. Among the recurrent cases, there were 10 high-risk cases and 7 cases treated with imatinib. There was no significant difference between the TA and NTA groups in terms of DFS and OS. The 3- and 5-year DFS rates were 81.3% and 65.1% for the TA group and 79.0% and 65.9% for the NTA group, respectively (P=0.243) (Figure 3). There were 4 deaths in the whole group, and 3 cases were high-risk cases. The 3- and 5-year OS rates were 95.0% and 88.7% for the TA group and 93.3% and 93.3% for the NTA group (P=0.308) (Figure 4). Univariate analysis showed that tumor size, mitotic count and NIH risk were the factors influencing DFS. However, multivariate analysis did not find any independent risk factors affecting DFS. For OS, univariate and multivariate analysis found no prognostic factors or independent risk factors (Table 5).
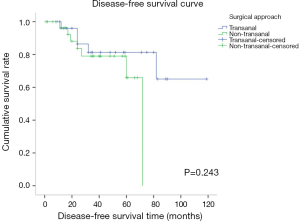
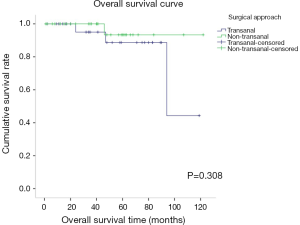
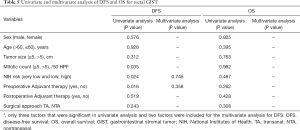
Full table
Discussion
This study was a single-center, retrospective study that collected 82 pathologically confirmed rectal GIST cases (Figure 1), accounting for 15.3% (82/537) of all GIST cases in a single-center, which is higher than the 4–5% reported in previous studies (2,7). This discrepancy may be because our center is one of the largest colorectal clinics in South China. As the current diagnosis and treatment of rectal GIST is still controversial and difficult, more rectal GIST cases may be admitted to our center. As in previous literature reports (13-16), the asymptomatic rate of patients with rectal GIST was between 9.5% and 36.2%. In our study, 23 cases (35.9%, 23/64) had no specific clinical symptoms before surgery, suggesting that rectal GIST is full of challenges in early diagnosis. Therefore, how to screen asymptomatic groups in a timely manner is the main effort for colorectal surgeons in the future. In this study, only 10 (15.6%, 10/64) cases performed a genetic test, which was lower than the 36.8% (7/19) and 34.0% (16/47) of cases reported by Wilkinson (11) and Cavnar (16). Genetic testing is an essential means in the era of precision therapy (17). The lower rate of genetic testing in our study may be related to the lower prevalence rate in the past and higher test costs.
Preoperative neoadjuvant treatment is a promising concept that has been successful in a variety of solid tumors (18-20). Preoperative neoadjuvant treatment can shrink the tumor, reduce the risk of subsequent surgery and the incidence of complications. Otherwise, preoperative neoadjuvant treatment can improve the R0 resection rate, verify the drug response and improve the prognosis (21). Previous studies (22) have shown that preoperative neoadjuvant therapy has a response rate (%) of 42–100%, a sphincter-preserving rate (%) of 33.3–100% and an R0 resection rate of 77.3–100%. The 5-year OS rate can reach 90%. In this study, 29 cases (45.3%, 29/64) were treated with preoperative neoadjuvant therapy. As reported in previous studies (11,13-16,23-25) (Table 6) on surgically resected rectal GIST, the neoadjuvant rate was 0–81.8%. Additionally, the preoperative neoadjuvant therapy in this study was significantly different between the TA and NTA groups (P=0.003), 7 cases in the TA group and 22 in the NTA group, which was similar to the study reported by Cavnar (16). This result is mainly related to the preoperative tumor size (15). Tumor size is an objective criterion for clinicians to assess whether preoperative neoadjuvant therapy is available. The tumor size in the NTA group was larger than that in the TA group (P=0.002) (Table 3). Thus, tumor size is the consideration for surgeons to choose a suitable surgical approach in the resection of rectal GIST (13,23,25). How to shrink the tumor by effective preoperative neoadjuvant treatment and choose a less traumatic surgical approach is the future direction of research.
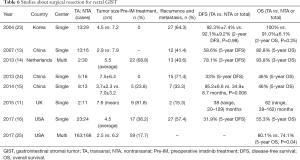
Full table
Based on the clinical practice and conclusions of related research, TA surgery has the advantages of small surgical trauma, high anus-preserver rate, high R0 resection rate, and low complication rate, so this strategy is one of the choices for the surgical resection of rectal GIST (11). Previous studies have shown that the rate of TA resection in all surgical cases is 6.3–48.9% (13-16,23,24). In this study, 29 cases underwent TA surgery, and 35 cases underwent NTA surgery. The TA resection rate accounted for 45.3% of all surgical resection cases. Higher rates of TA surgery may be related to advancements in surgical techniques and the development of surgical platforms and the role of preoperative neoadjuvant therapy. Previous studies have confirmed that TA surgery can achieve ideal DFS and OS for rectal GIST. In our study, we also observed that the operation time was shorter (P=0.013), the blood loss was less (P=0.038), the anus-preserver rate was higher (P=0.048), the 30-day complication rate was lower (P=0.000) and hospital stays were shorter (P=0.011) in the TA group. Therefore, if a good prognosis can be achieved, TA surgery is worthy of clinical promotion (11,26). As mentioned above, tumor size is the consideration for surgeons to choose the surgical approach, and the distance of the tumor from the anus is also a problem that surgeons need to consider. In this study, the distance from the anus was shorter in the TA group than in the NTA group (4.2±0.9 vs. 5.8±1.1, P=0.047), which is consistent with a previous study (15).
To evaluate the clinical value of a surgical approach, in addition to considering its safety and feasibility, it is also important to evaluate the impact of this approach on prognosis (16). In this study, although the tumor was larger, the mitotic count was higher and the number of high-risk cases was higher in the NTA group, but there was no significant difference between the two groups in DFS or OS, which may be related to the proportion of preoperative neoadjuvant therapy being higher and 70% (21/30) of the high-risk cases receiving postoperative adjuvant therapy in the NTA group. In the study of Liu (15), the TA group achieved a better DFS, and the results may be related to more high-risk cases in the NTA group but a lower proportion of adjuvant treatments (25%). Many previous studies have confirmed (8,27,28) that perioperative imatinib treatment can improve DFS and OS in patients with GIST.
In the previous discussion, our study demonstrated that distance from the anus and tumor size were important considerations for surgeons in choosing TA or NTA surgery. Therefore, we recommend that patients within 5 cm from the anus and with a tumor size of less than 5 cm may undergo TA surgery in an experienced center. However, in the context of the rapid development of TEM, TME, other surgical operating platforms and surgical energy instruments, sometimes distance from the anus and tumor size are not completely necessary indicators for surgeons to choose surgical methods (29-31). The limitation of this study is that it is a single-center, small-sample, retrospective study. However, this study is currently the largest sample of studies on the surgical approach to rectal GIST selection in China, which can provide some information and guidance for clinical practice.
Conclusions
TA surgery is an effective approach for the resection of rectal GIST because of its minimally invasive advantages, such as short operation time, less blood loss, rapid recovery, and low complication rate. In addition, TA surgery has an ideal rate of anal sphincter preservation, achieving a good DFS and OS, which is worthy of clinical promotion. However, this study is only a retrospective, single-center, small-sample study, and the conclusions still need to be confirmed by a prospective, multicenter, large-sample study. Moreover, how to select the appropriate rectal GIST cases for preoperative neoadjuvant therapy and improve the proportion of TA resection is the direction that still needs to be studied in the future.
Acknowledgments
Funding: This work was funded by the Natural Science Foundation of Guangdong Province grant (2017A030310407 to Wang Huaiming).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Research Ethics Committee, the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China (No. 2018ZSLYEC-099). Informed consent for participation in the study was obtained either directly, or from a guardian of each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus 2019;11:e4120. [PubMed]
- Yang Z, Feng X, Zhang P, et al. Clinicopathological features and prognosis of 276 cases of primary small (≤ 2 cm) gastric gastrointestinal stromal tumors: a multicenter data review. Surg Endosc 2019;33:2982-90. [Crossref] [PubMed]
- Kameyama H, Kanda T, Tajima Y, et al. Management of rectal gastrointestinal stromal tumor. Transl Gastroenterol Hepatol 2018;3:8. [Crossref] [PubMed]
- Ulanja MB, Rishi M, Beutler BD, et al. Racial disparity in incidence and survival for gastrointestinal stromal tumors (GISTs): an analysis of SEER database. J Racial Ethn Health Disparities 2019;6:1035-43. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:536-63. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281-93. [Crossref] [PubMed]
- Cavnar MJ, Seier K, Curtin C, et al. Outcome of 1000 patients with gastrointestinal stromal tumor (GIST) treated by surgery in the pre and post-imatinib eras. Ann Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Seita K, Yoneyama F, Kimura K, et al. Resection of an advanced rectal gastrointestinal stromal tumor following neoadjuvant chemotherapy. Gan To Kagaku Ryoho 2018;45:973-5. [PubMed]
- Wilkinson MJ, Fitzgerald JE, Strauss DC, et al. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg 2015;102:965-71. [Crossref] [PubMed]
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:758-86. [Crossref] [PubMed]
- Dong C, Jun-Hui C, Xiao-Jun Y, et al. Gastrointestinal stromal tumors of the rectum: clinical, pathologic, immunohistochemical characteristics and prognostic analysis. Scand J Gastroenterol 2007;42:1221-9. [Crossref] [PubMed]
- Tielen R, Verhoef C, van Coevorden F, et al. Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol 2013;107:320-3. [Crossref] [PubMed]
- Liu H, Yan Z, Liao G, et al. Treatment strategy of rectal gastrointestinal stromal tumor (GIST). J Surg Oncol 2014;109:708-13. [Crossref] [PubMed]
- Cavnar MJ, Wang L, Balachandran VP, et al. Rectal gastrointestinal stromal tumor (GIST) in the era of imatinib: organ preservation and improved oncologic outcome. Ann Surg Oncol 2017;24:3972-80. [Crossref] [PubMed]
- Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv68-78. [Crossref] [PubMed]
- Esen E, Karahasanoğlu T, Özben V, et al. Complete response after neoadjuvant treatment for rectal cancer. Lancet 2019;393:1694. [Crossref] [PubMed]
- Meng X, Wang L, Zhao Y, et al. Neoadjuvant chemoradiation treatment for resectable esophago-gastric cancer: a systematic review and meta-analysis. J Cancer 2019;10:192-204. [Crossref] [PubMed]
- Fasching PA, Hartkopf AD, Gass P, et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat 2019;173:319-28. [Crossref] [PubMed]
- Hou YY, Zhou Y, Lu SH, et al. Imatinib mesylate neoadjuvant treatment for rectal malignant gastrointestinal stromal tumor. World J Gastroenterol 2009;15:1910-3. [Crossref] [PubMed]
- Kaneko M, Emoto S, Murono K, et al. Neoadjuvant imatinib therapy in rectal gastrointestinal stromal tumors. Surg Today 2019;49:460-6. [Crossref] [PubMed]
- Changchien CR, Wu MC, Tasi WS, et al. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Dis Colon Rectum 2004;47:1922-9. [Crossref] [PubMed]
- Xiao CC, Zhang S, Wang MH, et al. Clinicopathological features and prognostic factors of rectal gastrointestinal stromal tumors. J Gastrointest Surg 2013;17:793-8. [Crossref] [PubMed]
- Hawkins AT, Wells KO, Krishnamurty DM, et al. Preoperative chemotherapy and survival for large anorectal gastrointestinal stromal tumors: a national analysis of 333 cases. Ann Surg Oncol 2017;24:1195-201. [Crossref] [PubMed]
- Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586-92. [Crossref] [PubMed]
- Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: the PERSIST-5 clinical trial. JAMA Oncol 2018;4:e184060. [Crossref] [PubMed]
- Laurent M, Brahmi M, Dufresne A, et al. Adjuvant therapy with imatinib in gastrointestinal stromal tumors (GISTs)-review and perspectives. Transl Gastroenterol Hepatol 2019;4:24. [Crossref] [PubMed]
- Pintor-Tortolero J, García JC, Cantero R. Transanal minimally invasive surgery approach for rectal GIST. Tech Coloproctol 2016;20:321-2. [Crossref] [PubMed]
- Hoshino N, Hida K, Kawada K, et al. Transanal total mesorectal excision for a large leiomyosarcoma at the lower rectum: a case report and literature review. Surg Case Rep 2017;3:13. [Crossref] [PubMed]
- Eldamshety O, Metwally IH, Ghoneem E, et al. Resection of rectal GIST using a novel technique: a report of two cases. Ecancermedicalscience 2017;11:760. [Crossref] [PubMed]