
Logistic regression analyses of factors affecting fertility of intrauterine adhesions patients
Introduction
Intrauterine adhesion (IUA), also called Asherman syndrome, was first reported and described by Joseph Asherman in 1948 (1). It is the partial, or complete, obliteration of a gravid, or non-gravid, uterine cavity by adhesions secondary to trauma (2). IUA results from an intrauterine injury which disrupts the endometrium basal layer.
Adhesions are abnormal fibrous connections in which vascular channels join tissue surfaces forming abnormal sites. They have a varied etiology (3). Adhesions, which impair the blood supply, can cause partial, or total, uterine cavity obliteration, reduce the size of the implantation area, and diminish endometrium receptivity. IUA typically presents with amenorrhea, hypomenorrhea, infertility, and repeat abortions (4,5). IUA prevalence is difficult to measure, but appears to have increased over the last few decades. This is probably due to an increase in iatrogenic endometrial trauma. Postpartum curettage and surgical abortions are considered major causes of IUA. Other causes include genital tuberculosis, pelvic irradiation, and uterine surgery (including hysteroscopic surgery) (6). Hooker et al. (7) reported curettage during pregnancy as a primary IUA cause.
Patients with severe IUA suffer long treatment periods, pay significant medical expenses, and have poor prognoses. This greatly impacts personal and family quality of life. IUA diagnosis and treatment is essential in evaluating infertile patients including those undergoing in vitro fertilization (IVF). Hysteroscopic adhesiolysis (HA) has been the gold standard treatment for IUA (8). Treatment outcomes have been improved dramatically as hysteroscopic techniques have developed.
IUAs can be dissected using hysteroscopic scissors or other methods, including L-hook electrodes or loop electrodes. HA aims to restore uterine cavity volume and shape to increase fertility potential (9). The procedure can be challenging (10), and patients undergoing surgery should be counseled regarding the possibility of a repeat surgery due to the high-risk of recurrence of adhesions, especially in a severe IUA (11-14). Various adjuvant therapies have been proposed to avoid IUA reformation. Post-procedure strategies include placing an intrauterine device (IUD) (15), Foley catheter balloon (5), fresh amnion grafts (16), and hyaluronic acid gel (17). Some of these interventions have decreased the likelihood of recurrence. Prevalence rates for women suffering from IUA recurrence after the use of various adjuvant therapies remain high.
Hysteroscopy is the gold standard for IUA diagnosis. Surgery is necessary (18-20). However, the reproductive outcomes following HA for moderate-severe IUAs [scores between 5 and 12 according to American Fertility Society (AFS) classification] were unsatisfactory, and few studies have analyzed the clinical characteristics pre-, intra- and post-HA to determine the main risk factors for infertility in patients with IUAs. Therefore, this study aimed to identify the risk factors for infertility and thereby provide guidance for improving the reproductive prognosis of patients with moderate-severe IUAs.
Methods
Patients
A total of 406 patients underwent HA at the Third Xiangya Hospital of Central South University between January 1st, 2016 and May 31st, 2017. Written informed voluntary consent was obtained. The study was approved by the Third Xiangya ethics committee. IUAs were scored by one surgeon applying the AFS classification system (21). IUAs were scored as follows: 1–4 (mild), 5–8 (moderate), and 9–12 (severe).
The inclusion criteria were as follows: (I) IUA confirmed by hysteroscopy; (II) a desire for fertility; and (III) normal hormone levels and ovulation. The exclusion criteria were as follows: (I) tubercular IUA; (II) presence of other intrauterine diseases such as endometrial polyps or atypical hyperplasia; and (III) grossly abnormal partner semen.
Of the 406 IUAs collected initially, 26 IUAs were excluded (9 patients lost to follow-up, 2 tubercular IUAs, 8 patients with a temporary lack of fertility desire, 7 patients with other infertility diseases). Finally, 380 patients met inclusion criteria. Only those 380 IUAs with follow-ups ranging from 2 to 3 years were included. Three-dimensional transvaginal ultrasound (3D-TVUS) was carried out from the 21st to the 25th day of the menstrual cycle. Data obtained from 3D-TVUS was used for intraoperative judgement during HA. The length of disease course, which was defined as the time from the last intrauterine surgery that caused IUAs to undergoing HA, was recorded for every patient. Medical records, intraoperative descriptions, and hysteroscopic images were reviewed.
Surgical procedure
HA was performed under intravenous anesthesia. Patients fasted 6–8 hours before surgery. Rectal misoprostol (400 mg) was administered 2 hours before surgery. Sterile saline solution was used to distend the uterus. Distension pressure was 110–120 mmHg with a flow rate of 300–350 mL/m. A diagnostic hysteroscopy with a 4.5 mm out sheath diameter explored the uterine cavity and evaluated the AFS adhesion scores prior to HA. The operation was monitored by transabdominal ultrasound. A 6.5 mm operative hysteroscope was used to perform HA after the cervical canal was dilated to 7.5 mm. Uterine cavity adhesions were separated using a 7 Fr rigid single-action scissors. Adhesiolysis was performed from the central portion to the bilateral walls, uterine horns, and uterus fundus. After the entire uterine cavity was restored, a uterine-shaped stainless-steel IUD was inserted into the uterine cavity with its position checked via hysteroscopy to ensure that the IUD size matched the uterine cavity size and that the IUD was correctly positioned (22,23). A double-channel, 12 Fr Foley catheter balloon, with the top catheter portion removed, was inserted into the uterine cavity and distended using 2.5 mL of sterile saline with the balloon in the center of the uterine-shaped IUD. A total of 3 mL hyaluronic acid gel was injected into the uterine cavity via the catheter (4). No complications were recorded.
Postoperative management and follow-up
The Foley catheter was removed 3 days after surgery in moderate IUAs and 7 days after for severe IUAs. Patients had monthly ultrasound scans to ensure the position of the IUD was normal after catheter removal. Hormone therapy commenced with estradiol valerate 3 mg bid for 21 days or following the patient’s menstrual cycle, and progesterone 100 mg Qn was added for the last 6 days of the menstrual cycle, for 3 cycles, to promote endometrial growth. A follow-up hysteroscopy was performed 1 month and 4 months after initial surgery for severe IUA patient, and 3 months after initial HA for moderate IUA patients until the uterine cavity was IUA-free or further improvement was likely impossible. If adhesions returned, adhesiolysis was performed. Otherwise, no surgery was performed. The IUD was removed during the final hysteroscopy.
It was suggested to patients that they resume conception efforts after successful adhesion-free HA or to abandon treatment if most of the uterine cavity could not be restored and further improvement was impossible. 3D-TVUS examinations were performed to measure the endometrial thickness and to evaluate the recurrence of IUAs every 3 months during the luteal phase on day 21 to 25 of the cycle. This is considered the optimal time to examine patients for the presence of uterine anomalies because the endometrium increases in volume, and the uterine cavity is stretched in the coronal plane. Endometrium can be clearly differentiated from the surrounding myometrium (24). All patients were followed up for at least 2 years post-surgery for pregnancy, spontaneous abortion, and live birth rates.
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System 9.4. Differences between pregnant and non-pregnant patients were tested using either a χ2, or Fisher’s exact test as appropriate. Logistic regression analysis was applied to determine which was the single dominant factor. A P<0.05 value was considered statistically significant.
Results
Of the 406 patients with IUA, 26 were lost during follow-up or excluded by other criteria. Only 380 were included. Of the 380, 215 (56.6%) became pregnant. Of this group, 18 spontaneously miscarried, 5 of the live births occurred prematurely between gestational weeks 31 to 36, 182 live births delivered at term, and 10 were still pregnant at the end of the study. Clinical characteristics of the 380 women with IUAs are shown in Table 1.
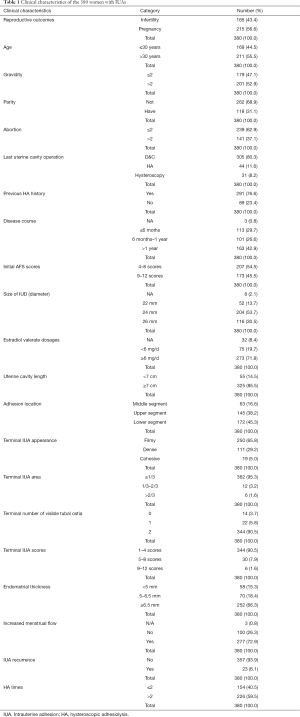
Full table
The variates including age, a prior history of uterine cavity operation, previous HA history, disease course, IUD size, uterine cavity length, adhesion location, number of visible tubal ostia, AFS scores, endometrial thickness, increased menstrual flow, IUA recurrence, and number of HAs, were significantly related to the pregnancy rate (P<0.05). The other variables were not statistically significant (P>0.05) (Table 2).
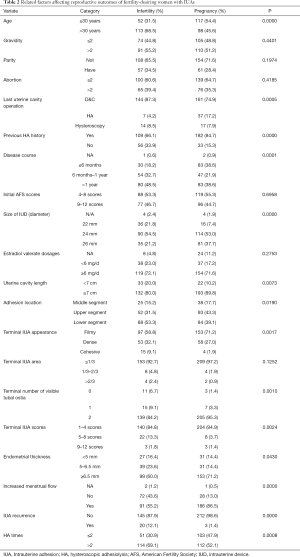
Full table
Univariate analysis evaluated the infertility risk factors (Table 3). Compared with the pregnant group, the infertile group had more patients aged >30 years (P<0.0001, OR =2.594, 95% CI: 1.697–3.964). Infertile patients were more likely: (I) to have had prior HA procedures (P<0.0001, OR =2.833, 95% CI: 1.734–4.631), (II) a longer disease course (6–12 months: P<0.0001, OR =3.179, 95% CI: 1.794–5.632; >12 months: P=0.0002, OR =2.667, 95% CI: 1.588–4.478), (III) a more cohesive adhesion case (P=0.0021, OR =5.915, 95% CI: 1.907–18.345), (IV) a more moderate IUA case (P=0.0012, OR =4.007, 95% CI: 1.735–9.257), (V) more IUA recurrence (P=0.0003, OR =9.739, 95% CI: 2.843–33.367), (VI) a lack of increased menstrual flow (P<0.0001, OR =0.19, 95% CI: 0.115–0.315), (VII) or HA procedures ≥2 times (P=0.0009, OR =2.056, 95% CI: 1.344–3.144) than pregnant patients. Other variables showed no difference between the two groups (P>0.05)
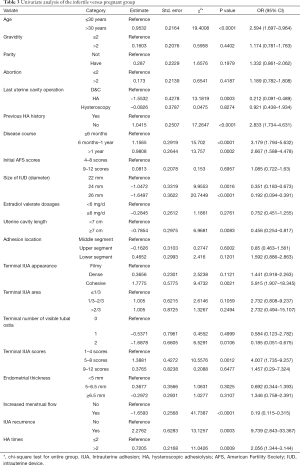
Full table
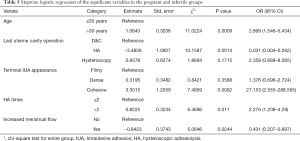
A multivariate logistical regression analysis was carried out based on the meaningful variables (P<0.05) of univariate logistical regression analysis. Full and stepwise regression methods were used in selecting model variables (Tables 4,5). Patients aged >30, a longer disease course, cohesive adhesion, no increased menstrual flow, and HA procedures ≥2 times, were factors significant to patient-related risk factors associated with the reproductive outcomes of fertility-desiring women with IUA in this study (P<0.05), but other variables were excluded in the model, as they did not have a significant relationship with the reproductive outcomes.
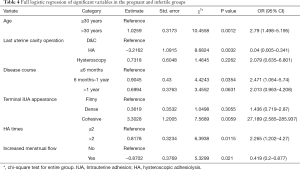
Full table
Full table
Discussion
As hysteroscopy has come to be used more often, especially in IUA diagnosis among women of childbearing age, its reported incidence has increased considerably (10,25). The most common risk factors associated with IUA are pregnancy-associated (26,27). Prior reports indicate that post-miscarriage dilation and curettage (D & C) account for about 90% of all IUAs (28). IUAs are not rare in China because the annual D & C rate is approximately 2.9% (29). In this study, 80.3% of the subjects had a history of D & C, while 8.2% had a history of hysteroscopic intrauterine lesion excision including endometrial polyps, uterus septum, and submucosal myoma. This finding was consistent with prior studies.
Previous studies have reported age, at the age divide of 30, as an important risk factor for infertility (30). Age-related decline in fertility has a greater impact on the cumulative live birth rate at older ages (31). The reason for this may be that there is a strong positive age-independent relationship with anti-Müllerian hormone (AMH) levels and ovarian reserve measurements by means of AMH which is highly relevant when counseling infertile patients (32). This study showed that age (>30 years) was a risk factor for infertility with a P value of <0.05 in both univariate and multivariate logistic regression models.
A previous report found that women suffering from IUA recurrence had a much lower pregnancy rate than those without (25). The main purpose of surgery is to achieve a normal cavity and facilitate fertility. Successful IUA treatment relies on a complete excision of adhesive tissues and aims to prevent recurrence. Reproductive outcomes are adversely affected by frequent adhesion recurrence (7). Comprehensive treatment is needed to achieve both a healthy uterine cavity and normal menstruation while limiting the number of surgeries needed to improve reproductive outcomes. In the current study, patients who underwent frequent adhesiolysis owing to repeated IUA recurrence had lower pregnancy rates than those who underwent only 1 or 2 operations. This is consistent with a previous study (7). Multiple surgeries may be necessary in some cases but may not always produce the desired outcome (33). In this study, IUA recurrence and HA, 2008es were high risk factors for infertility.
To our knowledge, there is little research concerning the impact of disease course on reproductive outcomes. In this study, infertile patients had a longer disease course than pregnant patients (P<0.05). The fertility benefits of a shorter disease course might be explained by time-related histological changes after endometrial trauma. According to a prospective cohort study, endometrial wound healing durations vary according to the type of pathology. It also may depend on endometrium recovery which may range from 1 month following the hysteroscopic removal of polyps to 3 months following hysteroscopic myomectomy (34). In this study, patients sought treatment in less than 6 months once clinical symptoms appeared after the latest D & C owing to routine treatment management.
The post-HA procedure in this study consisted of a uterine shaped stainless-steel IUD being inserted into the uterine cavity and monitored via hysteroscopy to ensure that the IUD size matched the uterine cavity size. IUD size is highly relevant to uterine cavity volume. A smaller uterine cavity volume and shorter length means it has contracted due to adhesion tissue. Removing uterine contracture could successfully increase uterine cavity volume and promote endometrium restoration which improves the likelihood of implantation along with the growth and development of the fertilized ovum (22). Gao et al. (35) reported that decreased uterine cavity volumes may result in delivery rate differences. Cenksoy et al. reported that the depth of the uterine cavity may be considered to indirectly be an important factor due to affecting the ET depth (36). In our study, the IUD size was an indicator related to pregnancy.
One previous report found depositing embryos in the uterine mid-fundal area to be valuable in improving pregnancy rates (37). A possible reason is that the fundal endometrium is suitable for implantation as there is a tendency to a lower endometrial wavelike activity and higher endometrial tissue blood flow in the fundal endometrium (38-40). IUA in the upper uterine cavity segment, particularly in the fundal area destroyed the most suitable implanting site which might have resulted in a decrease of pregnancy rates. The current study’s results agree with those of other researchers in that IUA in the upper uterine cavity segment, particularly in the fundal area, was found to be a significant risk factor for pregnancy.
It is generally accepted that fecundity decreases when the endometrium is <6.5 to 7 mm (41). Many women with IUA are unable to achieve significant endometrial growth even with prolonged estradiol supplementation (42). In this study, endometrial thickness had a significant relationship with pregnancy, but when all covariates were included either in the bivariate or binary logistic regression models, or in stepwise logistic regression analysis of all covariates, endometrial thickness was not an independent factor determining the reproductive outcome. As is known, functioning endometrium affect implantation and conceptus survival (43). Endometrial thickness was correlated with endometrial function but does not fully represent endometrial function which was changed with intrauterine microenvironment changing in IUAs.
A functioning endometrium significantly affects implantation and the period preceding it, as when a nonattached conceptus takes sustenance entirely from endometrial gland exocrine secretions (43). Endometrial gland secretion—histotroph or uterine milk—contains a multitude of proteins essential for conceptus survival, growth, and development during the early stages of pregnancy (44). These secretions are particularly important for the nourishment of the conceptus in the peri-implantation period before establishment of hemotrophic nutrition by the allantochorionic placenta (45). As menstruation recovery is determined by endometrial function, increased menstrual volume post-HA can be a potential predictor of restored endometrium function.
In conclusion, older age, longer disease courses, cohesive adhesion, no increased menstrual volume, and more numerous HA procedures, will have significantly negative impacts on the reproductive outcomes of IUA patients.
Acknowledgments
Funding: This study is supported by the Natural Science Foundation of China (Grant No. 81671492) and the Hunan Science and Technology Department (Grant No. 2018SK2102). B Gao is funded by China Scholarship Council (File number: 201806370178).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was given to the study by The Institutional Review Board (IRB) of Third Xiangya Hospital and Xiangya Hospital, Central South University. The procedure was performed in accordance with relevant guidelines and regulations. Informed consent was obtained after the procedure was fully explained to all participants and their legal guardians.
References
- Asherman JG. Amenorrhoea traumatica (atretica). J Obstet Gynaecol Br Emp 1948;55:23-30. [Crossref] [PubMed]
- Tan IF, Robertson M. The role of imaging in the investigation of Asherman's syndrome. Australas J Ultrasound Med 2011;14:15-8. [Crossref] [PubMed]
- Sugimoto O. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions. Am J Obstet Gynecol 1978;131:539-47. [Crossref] [PubMed]
- Salma U, Xue M, Md Sayed AS, et al. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomed Res Int 2014;2014:589296. [Crossref] [PubMed]
- Chen Y, Liu L, Luo Y, et al. Effects of Aspirin and Intrauterine Balloon on Endometrial Repair and Reproductive Prognosis in Patients with Severe Intrauterine Adhesion: A Prospective Cohort Study. Biomed Res Int 2017;2017:8526104. [PubMed]
- Gan L, Duan H, Xu Q, et al. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017;19:603-16. [Crossref] [PubMed]
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014;20:262-78. [Crossref] [PubMed]
- Yamamoto N, Takeuchi R, Izuchi D, et al. Hysteroscopic adhesiolysis for patients with Asherman's syndrome: menstrual and fertility outcomes. Reprod Med Biol 2013;12:159-66. [Crossref] [PubMed]
- AAGL practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol 2010;17:1-7. [Crossref] [PubMed]
- Yu D, Wong YM, Cheong Y, et al. Asherman syndrome--one century later. Fertil Steril 2008;89:759-79. [Crossref] [PubMed]
- Pabuçcu R, Atay V, Orhon E, et al. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil Steril 1997;68:1141-3. [Crossref] [PubMed]
- Preutthipan S, Linasmita V. Reproductive outcome following hysteroscopic lysis of intrauterine adhesions: a result of 65 cases at Ramathibodi Hospital. J Med Assoc Thai 2000;83:42-6. [PubMed]
- Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol 1988;158:1459-70. [Crossref] [PubMed]
- Capella-Allouc S, Morsad F, Rongières-Bertrand C, et al. Hysteroscopic treatment of severe Asherman's syndrome and subsequent fertility. Hum Reprod 1999;14:1230-3. [Crossref] [PubMed]
- Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet 2003;82:49-56. [Crossref] [PubMed]
- Amer MI, Abd-El-Maeboud KH. Amnion graft following hysteroscopic lysis of intrauterine adhesions. J Obstet Gynaecol Res 2006;32:559-66. [Crossref] [PubMed]
- Acunzo G, Guida M, Pellicano M, et al. Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a prospective, randomized, controlled study. Hum Reprod 2003;18:1918-21. [Crossref] [PubMed]
- Yang JH, Chen MJ, Chen CD, et al. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil Steril 2013;99:2092-6.e3. [Crossref] [PubMed]
- Yang JH, Chen MJ, Wu MY, et al. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil Steril 2008;89:1254-9. [Crossref] [PubMed]
- Yang JH, Chen MJ, Chen CD, et al. Impact of submucous myoma on the severity of anemia. Fertil Steril 2011;95:1769-72.e1. [Crossref] [PubMed]
- Lin XN, Zhou F, Wei ML, et al. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil Steril 2015;104:235-40. [Crossref] [PubMed]
- Zhang A, Jamail G, Xue M, et al. Hysteroscopic Intrauterine Adhesiolysis Using the "Ploughing" Technique With Cold Scissors. J Minim Invasive Gynecol 2015;22:934-5. [Crossref] [PubMed]
- Huang H, Cheng C, Johnson G, et al. Hysteroscopic Intrauterine Adhesiolysis Using a Blunt Spreading Dissection Technique With Double-action Forceps. J Minim Invasive Gynecol 2018;25:583-4. [Crossref] [PubMed]
- Saravelos SH, Li TC. Intra-cycle variation of the uterine cavity indentation assessed with three-dimensional ultrasound in natural and stimulated cycles. Reprod Biomed Online 2016;32:545-50. [Crossref] [PubMed]
- Yu D, Li TC, Xia E, et al. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril 2008;89:715-22. [Crossref] [PubMed]
- March CM. Management of Asherman's syndrome. Reprod Biomed Online 2011;23:63-76. [Crossref] [PubMed]
- Takai IU, Kwayabura AS, Ugwa EA, et al. A 10-year Review of the Clinical Presentation and Treatment Outcome of Asherman's Syndrome at a Center with Limited Resources. Ann Med Health Sci Res 2015;5:442-6. [Crossref] [PubMed]
- Sharma JB, Roy KK, Pushparaj M, et al. Genital tuberculosis: an important cause of Asherman's syndrome in India. Arch Gynecol Obstet 2008;277:37-41. [Crossref] [PubMed]
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol 2010;17:555-69. [Crossref] [PubMed]
- Maheshwari A, Hamilton M, Bhattacharya S. Effect of female age on the diagnostic categories of infertility. Hum Reprod 2008;23:538-42. [Crossref] [PubMed]
- Goldman RH, Farland LV, Thomas AM, et al. The combined impact of maternal age and body mass index on cumulative live birth following in vitro fertilization. Am J Obstet Gynecol 2019;221:617.e1-13. [Crossref] [PubMed]
- La Marca A, Minasi MG, Sighinolfi G, et al. Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril 2017;108:777-83.e2. [Crossref] [PubMed]
- Robinson JK, Colimon LM, Isaacson KB. Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman's syndrome). Fertil Steril 2008;90:409-14. [Crossref] [PubMed]
- Yang JH, Chen CD, Chen SU, et al. The influence of the location and extent of intrauterine adhesions on recurrence after hysteroscopic adhesiolysis. BJOG 2016;123:618-23. [Crossref] [PubMed]
- Gao H, Liu DE, Li Y, et al. Uterine size and volume are associated with a higher clinical pregnancy rate in patients undergoing assisted reproduction technology: A longitudinal study (A STROBE-compliant article). Medicine (Baltimore) 2019;98:e14366. [Crossref] [PubMed]
- Cenksoy PO, Fıcıcıoglu C, Yesiladali M, et al. The importance of the length of uterine cavity, the position of the tip of the inner catheter and the distance between the fundal endometrial surface and the air bubbles as determinants of the pregnancy rate in IVF cycles. Eur J Obstet Gynecol Reprod Biol 2014;172:46-50. [Crossref] [PubMed]
- Madani T, Ashrafi M, Abadi AB, et al. Appropriate timing of uterine cavity length measurement positively affects assisted reproduction cycle outcome. Reprod Biomed Online 2009;19:734-6. [Crossref] [PubMed]
- Lambers MJ, Dogan E, Lens JW, et al. The position of transferred air bubbles after embryo transfer is related to pregnancy rate. Fertil Steril 2007;88:68-73. [Crossref] [PubMed]
- van Gestel I. Endometrial wave-like activity in the non-pregnant uterus. Hum Reprod Update 2003;9:131-8. [Crossref] [PubMed]
- Jinno M, Ozaki T, Iwashita M, et al. Measurement of endometrial tissue blood flow: a novel way to assess uterine receptivity for implantation. Fertil Steril 2001;76:1168-74. [Crossref] [PubMed]
- Isaacs JD, Wells CS, Williams DB, et al. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril 1996;65:262-6. [PubMed]
- Myers EM, Hurst BS. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril 2012;97:160-4. [Crossref] [PubMed]
- Malhotra N, Bahadur A, Kalaivani M, et al. Changes in endometrial receptivity in women with Asherman's syndrome undergoing hysteroscopic adhesiolysis. Arch Gynecol Obstet 2012;286:525-30. [Crossref] [PubMed]
- Stewart F, Gerstenberg C, Suire S, et al. Immunolocalization of a novel protein (P19) in the endometrium of fertile and subfertile mares. J Reprod Fertil Suppl 2000.593-9. [PubMed]
- Suire S, Stewart F, Beauchamp J, et al. Uterocalin, a lipocalin provisioning the preattachment equine conceptus: fatty acid and retinol binding properties, and structural characterization. Biochem J 2001;356:369-76. [Crossref] [PubMed]


