
Physical activity and laryngeal cancer
Introduction
Laryngeal cancer [International Classification of Diseases (ICD)-10 code C32; malignant neoplasms of larynx, including glottis, supraglottis, subglottis, and laryngeal cartilage], belongs to the specific category of head and neck malignancies and is a relatively rare condition. According to the Global Burden of Disease Cancer Collaboration, the currently estimated incidence of laryngeal cancer is 211 [95% confidence interval (95% CI), 206–216] per 1,000, with a 5:1 male to female ratio and approximately 10% of patients in metastatic or terminal phase (1). The estimated mortality for laryngeal cancer approximates 126 (95% CI, 123–130) per 1,000, again with a 5:1 male to female ratio. Notably, the burden of this malignancy, expressed as years lived with disability (YLDs), has increased by nearly 25% (25.1%; 95% CI, 21.7–28.5%) during the past 3 decades (2). The large majority of larynx cancers, approximating 98%, are represented by squamous cell carcinoma, whilst leiomyosarcomas, chondrosarcomas, lymphomas, and melanomas account for the remaining 2% (3).
According to the joint document of the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) (4), the most important factors associated with an enhanced risk of developing mouth, pharynx and larynx cancers include alcohol intake, tobacco (smoking, chewing and snuffing), human papillomavirus (HPV) infection, obesity (i.e., being overweight) and environmental exposure to asbestos, whilst some evidence exists that consuming non-starchy vegetables, choosing healthy dietary patterns, consuming coffee and avoiding mate may reduce the risk for this cancer. Notably, the WCRF and AICR also claim that limited evidence exists on the relationship between physical activity (PA) and mouth, pharynx and larynx cancers, so that no conclusions or recommendations can be made other than endorsing regular engagement in PA for preventing all other types of cancer. The American Cancer Society (ACS) clearly states that tobacco is the most important risk factor for larynx and hypopharynx cancers (5), followed by moderate or heavy alcohol use (i.e., >1 drink per day), HPV infection, exposure to asbestos or wood dust, paint fumes and chemicals used in petroleum, plastics, metalworking and textile industries, poor nutrition (i.e., vitamin deficiencies), intake of large amount of fried and processed foods, gastroesophageal reflux disease, inherited syndromes such as Fanconi anemia, and dyskeratosis congenita. Neither in this case, the ACS provides clear recommendations about favorable effects of PA on decreasing the risk of development and/or progression of laryngeal cancer.
Establishing whether PA recommendations are also valid for laryngeal cancer is pivotal, considering the accumulating evidence that PA may prevent the development of head and neck cancers, in general (6-8). Therefore, we aimed to review the current epidemiologic evidence on the effect of PA on larynx cancer risk to provide evidence-based, larynx cancer-specific indications.
Epidemiological evidence between PA and laryngeal cancer
The very first study which explored the relationship between PA and the risk of developing larynx cancer was published in 1993 by Dosemeci et al. (9). In this hospital-based study population, the PA levels performed by 779 patients diagnosed with larynx cancer were compared with that of 2,371 controls. The level of PA was defined as sedentary (<8 kJ/min), or working in jobs with moderate (between 8–12 kJ/min) and high (>12 kJ/min) intensity. After adjustment for age, smoking and socioeconomic status, sedentary subjects exhibited a non-significant trend (P=0.09) towards enhanced risk of laryngeal cancer compared to highly active subjects (OR, 1.2; 95% CI, 0.9–1.9), whilst the risk of moderately highly active subjects was globally comparable to that of highly active subjects (OR, 1.0; 95% CI, 0.8–1.2). A similar trend was observed for physical inactivity, i.e., when subjects seated for <2 h/day were compared to those seated for 2–6 h/day (OR, 1.1; 95% CI, 0.9–1.4) or for >6 h/day (OR, 1.1; 95% CI, 0.8–1.8; P=0.07 for trend).
The largest study on the relationship between laryngeal cancer and PA has been published by Leitzmann, in 2008, who followed-up as many as 487,732 subjects (192,479 women and 295,253 men) for a mean period of 7.2 years (10). A questionnaire was disseminated to obtain information on PA, whose level was ranked according to the frequency, i.e., <1, 1–2, 3–4 or ≥5 times per week. A total number of 406 individuals developed laryngeal cancer during follow-up. In univariate analysis, increasing level of PA was found to be inversely associated with the risk of developing cancer. More specifically, compared to sedentary subjects, the risk of developing larynx cancer was non-significantly decreased [relative risk (RR), 0.81; 95% CI, 0.59–1.11] in subjects exercising <1 time per week, but was significantly reduced by ~40% (RR, 0.61; 95% CI, 0.45–0.82) in those exercising 1–2 times per week and by ~50% in those exercising 3–4 times per week (RR, 0.54, 95% CI, 0.41–0.72) and ≥5 times per week (RR, 0.52; 95% CI, 0.38–0.71; P<0.001 for trend). In the subsequent multivariable analysis, the favorable effect of PA on the risk of developing laryngeal cancer was almost lost (P=0.225 for trend), whereby the RR compared to sedentary people was 0.96 (95% CI, 0.69–1.32), 0.82 (95% CI, 0.60–1.10), 0.84 (95% CI, 0.63–1.12) and 0.82 (95% CI, 0.59–1.13) in subjects exercising <1, 1–2, 3–4 or ≥5 times per week, respectively.
In a subsequent investigation, Nicolotti et al. (11) pooled data from four case-control studies including 612 cases of laryngeal cancer and 4,947 controls. PA was ranked as none/low (≤3 h per week or ≤1 time per week), moderate (2–9 h per week or 1–4 times per week) and high (any value above moderate) according to the frequency of practice in the four different studies, despite levels occasionally overlap. Notably, a highly significant, graded and inverse relationship could be observed between the level of PA and the risk of developing pharyngeal cancer. Compared to those reporting none/low PA, the risk of those reporting moderate PA was 33% lower (OR, 0.67; 95% CI, 0.53–0.85), whilst that of those reporting high PA was 42% lower (OR, 95% CI, 95% CI, 0.38–0.89). Unlike these findings, no relationship could be observed between the level of PA and the risk of developing laryngeal cancer. Compared to those reporting none/low PA, the risk of those reporting moderate PA was slightly but not significantly lower (OR, 0.81; 95% CI, 0.60–1.11), whilst that of those reporting high PA was significantly higher (OR, 1.73; 95% CI, 1.04–2.88). This last finding was partially explained by the authors with a higher number of smokers in their high PA group.
Lin et al. (12) carried out another case-control study involving 74 patients with laryngeal cancer and 731 healthy controls. Recreational PA was ranked according to lifestyle habits (sedentary or performing regular PA), intensity (sedentary, light, moderate and vigorous) and frequency (sedentary, 3 days per week, 4–5 days per week, 6–7 days per week). Compared to sedentary subjects, the risk of laryngeal cancer was found to be virtually identical in people reporting PA habits (OR, 1.03; 95% CI, 0.58–1.85), in those performing moderate (OR, 0.93; 95% CI, 0.46–1.87) or high (OR, 1.01; 95% CI, 0.45–2.24) volumes of PA, as well as in those exercising 3 days per week (OR, 1.19; 95% CI, 0.36–3.96), 4–5 days per week (OR, 0.32; 95% CI, 0.04–2.55) and even 6–7 days per week (OR, 1.14; 95% CI, 0.61–2.12).
Bravi et al. (13) pooled data from two Italian case-control studies including 689 patients with larynx cancer and 1,605 matched controls. Information on the level of PA was garnered by using a structured questionnaire, where participants self-reported occupational PA (i.e., very heavy, heavy, intermediate, standing and sedentary job) and leisure-time PA (hours of sports practice per week). Regarding the overall amount of PA, 1 point was attributed to people meeting the minimum amount (i.e., 150 min of moderate PA per week or 75 min of vigorous PA per week), 0.5 points to those who partially met the minimum amount, and a score of 0 when the minimum amount was largely unmet. Compared to subjects scoring 0 points, those with a score of 0.5 (OR, 0.58; 95% CI, 0.43–0.78; P=0.005) and 1 (OR, 0.61; 95% CI, 0.46–0.82; P=0.005) had nearly 40% lower risk of developing laryngeal cancer. Interestingly, a similar beneficial effect of PA was found also on the risk of developing overall head and neck cancers (between 26–30% reduction; P=0.001).
More recently, Kim et al. (14) performed a population-based retrospective study in South Korea including as many as 23.2 million people, 5,322 of whom were diagnosed with laryngeal cancer during a 7-year follow-up period. Notably, sedentary status (i.e., no routine PA) was reported by 54.9% of laryngeal cancer patients compared to 50.4% controls, so that PA was found to be associated with 16% decreased risk of larynx cancer (OR, 0.84; 95% CI, 0.79–0.88; P<0.001).
Another case-control study has been published (in Chinese) by Wang et al. (15). Although the contents of this article are not accessible, the authors concluded that PA may decrease the risk of laryngeal cancer by approximately 60% (OR, 0.41).
Discussion
Both PA and inactivity are believed to positively or negatively affect cellular processes and tumor growth. Briefly, PA reduces the risk of recurrence and improves survival throughout eliciting changes in adiposity, circulating levels of both adipokines and sex hormones, metabolic dysregulation, oxidative stress causing DNA damage and gene mutations, inflammation, and immune function (16-18). Moreover, PA might decrease the risk of cancer through epigenetic mechanisms (19).
Several biological mechanisms are implicated in the beneficial effects of exercise in cancer prevention and/or as adjuvant therapy (20). PA may prevent certain types of cancer as well as improve the quality of life among cancer survivors and/or those diagnosed with several malignancies, especially breast, gastrointestinal, colorectal, prostate, and lung cancers (21-23). This is also confirmed by the evidence that high-level athletes have a reduced risk of developing certain types of cancer (24). We previously suggested the combination of obesity, physical inactivity, and low fitness as a toxic triad highly implicated in cancer incidence and mortality (25), whilst physical inactivity itself is also related with an increased incidence of colorectal and breast cancer (26,27).
Taken together, the currently available scientific evidence seemingly attests that the impact of PA may be not so straightforward in lowering the laryngeal cancer risk as for other malignancies. In particular, reasonable consensus has been reached that moderate-intensity PA may generate the larger potential benefits on the risk of developing laryngeal cancer, whilst the effect of high-intensity PA is more contradictory, as shown in Table 1. In this regard, Nicolotti et al. (11) reported that high levels of PA might even be associated with an enhanced risk of developing this type of cancer. This finding is in keeping with the association between laryngeal malignancies and leanness. In particular, Garavello et al. (28) reported that subjects (especially men) with lower abdominal fat display a significantly enhanced risk of laryngeal cancer. This evidence has then been confirmed in subsequent studies (29,30). It is hence conceivable that part of the benefits conferred by high-regimen PA on lowering the general risk of cancers may be attenuated in the setting of laryngeal malignancies, whereby frequent, vigorous and strenuous exercise is inherently associated with leanness (31).
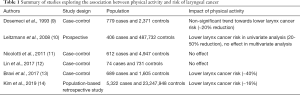
Full table
Despite the still controversial epidemiologic evidence, some plausible biological grounds would make it reasonable to expand PA and exercise recommendations to also embrace the prevention and care of laryngeal cancer. In the nationwide cohort study of Kim et al. (14), patients with metabolic syndrome had 13% enhanced risk of larynx cancer than those without metabolic syndrome. The role of PA for preventing and managing metabolic syndrome is extensively known (32). Even more importantly, current evidence advocates that adults should be engaged in 60 min of daily moderate-intensity PA for preventing unhealthy weight gain, thus reducing the risk of obesity and metabolic syndrome (33). This would hence contribute to explain the favorable association of moderate PA in lowering the larynx cancer risk.
The synergistic effect between low PA and smoking may provide an alternative explanation. In a large study including more than one hundred thousand individuals, O’Donovan et al. (34) showed that the combined risk of mortality in current cigarette smokers was indeed higher in sedentary people (OR, 3.38; 95% CI, 2.76–4.13) than in physically active individuals, and that same risk was very similar in those engaged in moderate (<60 min/week; OR, 2.35; 95% CI, 1.74–3.19) or high (>60 min/week; OR, 2.27; 95% CI, 1.64–2.1) levels of PA. Notably, cardiovascular mortality was paradoxically higher in subjects engaged in high (OR, 2.58; 95% CI, 1.33–5.03) than in those reporting moderate PA (OR, 2.45; 95% CI, 1.30–4.64). When the intensity of PA was expressed in terms of relative intensity, subjects engaged in middle-intensity exercise [6.63–23.8 metabolic equivalent (MET)/h per week; OR 0.87; 95% CI, 0.73–1.03] had a lower risk of cancer mortality than those engaged in high-intensity exercise (>23.8 MET/h per week; OR, 0.97; 95% CI, 0.81–1.16).
Another plausible mechanism encompasses the link between PA and inflammation. Vigorous, high-intensity physical exercise is always accompanied by an acute phase response, which is directly proportional to the intensity and duration of the physical effort, according to an exercise-dose-dependent manner (35-37). Since some pro-inflammatory conditions are associated with enhanced risk and faster progression of laryngeal cancer (38-41), it is hence reasonable to envision that moderate PA would produce large benefits than high-intensity exercise. Finally, emerging evidence attests that recreational PA may reduce the risk of gastroesophageal reflux disease (42), which is, in turn, a risk factor for laryngeal carcinogenesis (43).
Besides these important effects, recent literature data reveal that PA may play an important role in improving the quality of life, functional wellbeing, cardiorespiratory fitness, and self-esteem of patients with laryngeal cancer (44-47), thus further strengthening the inverse relationship between active lifestyle and laryngeal malignancies. Avoidance of sedentary behaviors may also be a relatively easy and important healthcare intervention for prolonging life expectancy in patients surviving from larynx cancer, whereby the 5-, 10- and 20-year cumulative risk of second primary laryngeal malignancies is as high as 4.6%, 9.9% and 19.0%, respectively (48).
Conclusions
In conclusion, the available clinical and biological evidence would lead us to conclude that promotion of an active lifestyle, characterized by performance of moderate-intensity PA (e.g., between 3 and 6 MET, equaling short distance running) may be beneficial for lowering the risk of developing laryngeal cancer as well as for improving the quality of life of cancer survivors. This specific training regimen seems to be even more effective than high-intensity exercise in individuals at a particularly higher risk of this type of malignancy (49).
Acknowledgments
Fabian Sanchis-Gomar is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent - Contractes Postdoctorals de la Universitat de València.”
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019. [Epub ahead of print]. [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Ciolofan MS, Vlaescu AN, Mogoanta CA, et al. Clinical, Histological and Immunohistochemical Evaluation of Larynx Cancer. Curr Health Sci J 2017;43:367-75. [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and cancers of the mouth, pharynx and larynx. 2018. Available online: . Accessed Octuber 29 2019.https://www.wcrf.org/dietandcancer
- American Cancer Society. Laryngeal and Hypopharyngeal Cancer Causes, Risk Factors, and Prevention. Available online: . Accessed October 29 2019.https://www.cancer.org/cancer/laryngeal-and-hypopharyngeal-cancer/causes-risks-prevention/risk-factors.html
- Hashibe M, Hunt J, Wei M, et al. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck 2013;35:914-22. [Crossref] [PubMed]
- Moore SC, Lee IM, Weiderpass E, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016;176:816-25. [Crossref] [PubMed]
- Platek AJ, Cannioto RA, Etter JL, et al. The association of lifetime physical inactivity with head and neck cancer: a hospital-based case-control analysis. Eur Arch Otorhinolaryngol 2017;274:3773-80. [Crossref] [PubMed]
- Dosemeci M, Hayes RB, Vetter R, et al. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control 1993;4:313-21. [Crossref] [PubMed]
- Leitzmann MF, Koebnick C, Freedman ND, et al. Physical activity and head and neck cancer risk. Cancer Causes Control 2008;19:1391-9. [Crossref] [PubMed]
- Nicolotti N, Chuang SC, Cadoni G, et al. Recreational physical activity and risk of head and neck cancer: a pooled analysis within the international head and neck cancer epidemiology (INHANCE) Consortium. Eur J Epidemiol 2011;26:619-28. [Crossref] [PubMed]
- Lin CL, Lee WT, Ou CY, et al. Regular recreational physical activity and risk of head and neck cancer. BMC Cancer 2017;17:286. [Crossref] [PubMed]
- Bravi F, Polesel J, Garavello W, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and head and neck cancers risk. Oral Oncol 2017;64:59-64. [Crossref] [PubMed]
- Kim SY, Han KD, Joo YH. Metabolic Syndrome and Incidence of Laryngeal Cancer: A Nationwide Cohort Study. Sci Rep 2019;9:667. [Crossref] [PubMed]
- Wang C, Li Q, Wang Y, et al. Case-control study on risk factors of laryngeal cancer in Heilongjiang province. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011;25:1117-9. [PubMed]
- Friedenreich CM, Shaw E, Neilson HK, et al. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl) 2017;95:1029-41. [Crossref] [PubMed]
- Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc 2019;51:2391-402. [Crossref] [PubMed]
- Sanchis-Gomar F, Lucia A, Yvert T, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila) 2015;8:105-10. [Crossref] [PubMed]
- Sanchis-Gomar F, Garcia-Gimenez JL, Perez-Quilis C, et al. Physical exercise as an epigenetic modulator: Eustress, the "positive stress" as an effector of gene expression. J Strength Cond Res 2012;26:3469-72. [Crossref] [PubMed]
- Brown JC, Winters-Stone K, Lee A, et al. Cancer, physical activity, and exercise. Compr Physiol 2012;2:2775-809. [PubMed]
- Luan X, Tian X, Zhang H, et al. Exercise as a prescription for patients with various diseases. J Sport Health Sci 2019;8:422-41. [Crossref] [PubMed]
- Sanchis-Gomar F. The skeletal muscle-metabolism axis in prostate-cancer therapy. N Engl J Med 2013;367:2257-8. [PubMed]
- Danese E, Salvagno GL, Tarperi C, et al. Middle-distance running acutely influences the concentration and composition of serum bile acids: Potential implications for cancer risk? Oncotarget 2017;8:52775-82. [Crossref] [PubMed]
- Garatachea N, Santos-Lozano A, Sanchis-Gomar F, et al. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin Proc 2014;89:1195-200. [Crossref] [PubMed]
- Sanchis-Gomar F, Lucia A, Pareja-Galeano H. Cancer Is Essentially Due to Bad Luck .. But Not So Much If You Don't Exercise or Are Overweight. Nutr Cancer 2015;67:865. [Crossref] [PubMed]
- Mattiuzzi C, Sanchis-Gomar F, Lippi G. Measuring the potential impact of physical inactivity on worldwide epidemiology of colorectal and breast cancers. Ann Cancer Epidemiol 2019. [Epub ahead of print]. [Crossref]
- Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med 2019;7:609. [Crossref]
- Garavello W, Randi G, Bosetti C, et al. Body size and laryngeal cancer risk. Ann Oncol 2006;17:1459-63. [Crossref] [PubMed]
- Gaudet MM, Olshan AF, Chuang SC, et al. Body mass index and risk of head and neck cancer in a pooled analysis of case-control studies in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int J Epidemiol 2010;39:1091-102. [Crossref] [PubMed]
- Etemadi A, O'Doherty MG, Freedman ND, et al. A prospective cohort study of body size and risk of head and neck cancers in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev 2014;23:2422-9. [Crossref] [PubMed]
- Jakicic JM. The effect of physical activity on body weight. Obesity (Silver Spring) 2009;17 Suppl 3:S34-8. [Crossref] [PubMed]
- Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab 2007;32:76-88. [Crossref] [PubMed]
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2013;1281:141-59. [Crossref] [PubMed]
- O'Donovan G, Hamer M, Stamatakis E. Relationships between exercise, smoking habit and mortality in more than 100,000 adults. Int J Cancer 2017;140:1819-27. [Crossref] [PubMed]
- Sanchis-Gomar F, Lippi G. Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 2014;24:68-79. [Crossref] [PubMed]
- Lippi G, Bassi A, Guidi G, et al. Relation between regular aerobic physical exercise and inflammatory markers. Am J Cardiol 2002;90:820. [Crossref] [PubMed]
- Niemela M, Kangastupa P, Niemela O, et al. Acute Changes in Inflammatory Biomarker Levels in Recreational Runners Participating in a Marathon or Half-Marathon. Sports Med Open 2016;2:21. [Crossref] [PubMed]
- Shivappa N, Hebert JR, Rosato V, et al. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer Causes Control 2016;27:1027-34. [Crossref] [PubMed]
- Shi J, Leng W, Zhao L, et al. Nonsteroidal anti-inflammatory drugs using and risk of head and neck cancer: a dose-response meta analysis of prospective cohort studies. Oncotarget 2017;8:99066-74. [Crossref] [PubMed]
- Zeng YC, Chi F, Xing R, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with locoregionally advanced laryngeal carcinoma treated with chemoradiotherapy. Jpn J Clin Oncol 2016;46:126-31. [PubMed]
- Du J, Liu J, Zhang X, et al. Pre-treatment neutrophil-to-lymphocyte ratio predicts survival in patients with laryngeal cancer. Oncol Lett 2018;15:1664-72. [PubMed]
- Lam S, Hart AR. Does physical activity protect against the development of gastroesophageal reflux disease, Barrett's esophagus, and esophageal adenocarcinoma? A review of the literature with a meta-analysis. Dis Esophagus 2017;30:1-10. [Crossref] [PubMed]
- Vaezi MF, Qadeer MA, Lopez R, et al. Laryngeal cancer and gastroesophageal reflux disease: a case-control study. Am J Med 2006;119:768-76. [Crossref] [PubMed]
- Midgley AW, Lowe D, Levy AR, et al. Exercise program design considerations for head and neck cancer survivors. Eur Arch Otorhinolaryngol 2018;275:169-79. [Crossref] [PubMed]
- Capozzi LC, Nishimura KC, McNeely ML, et al. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: a systematic review. Br J Sports Med 2016;50:325-38. [Crossref] [PubMed]
- Sammut L, Ward M, Patel N. Physical activity and quality of life in head and neck cancer survivors: a literature review. Int J Sports Med 2014;35:794-9. [Crossref] [PubMed]
- McNeely ML. Exercise as a promising intervention in head & neck cancer patients. Indian J Med Res 2013;137:451-3. [PubMed]
- Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer 2008;123:2390-6. [Crossref] [PubMed]
- Douma JAJ, Verdonck-de Leeuw IM, Leemans CR, et al. Demographic, clinical and lifestyle-related correlates of accelerometer assessed physical activity and fitness in newly diagnosed patients with head and neck cancer. Acta Oncol 2019.1-9. [Epub ahead of print]. [Crossref] [PubMed]


