Ex vivo lung perfusion with perfusate purification for human donor lungs following prolonged cold storage
Introduction
Lung transplantation (LTx) provides the only effective treatment option for selected patients with end-stage lung diseases. Although the number of potential donors is limited, the primary cause of this shortage is the approximately 80% of potential donor lungs that are unusable for standard LTx due to irreversible pre-existing lung disease or reversible damage occurring during end-of-life care (1). Since Steen reported the first ex vivo lung perfusions (EVLP) on donations after circulatory death (DCD) donor assessment and successfully performed LTx in 2001 (2), EVLP has been used as the most effective technique for estimating marginal donor lungs.
There are three protocols commonly used in EVLP at present: the Toronto protocol, the Organ Care System (OCS) protocol, and the Lund protocol. Other than a few case reports (3,4), EVLP is still mainly used as a donor evaluation tool in clinic, but not as an approach to repair injured lungs, while its high running cost limits the use of EVLP in clinic. Thus, having longer running times and lower running costs is necessary for EVLP to develop into an effective reparative procedure.
As in most other LTx centers, we established EVLP in the laboratory according to the Toronto protocol to complete the preservation model of porcine lung, and found that the installation of dialyzer for perfusion filtration could also maintain a good oxygen supply and capacity of EVLP for 4 hours. This study attempted to extend the EVLP running time to 12 hours with a dialyzer rather than replacing the perfusate periodically; the subsequent effects on lung function, pulmonary edema, inflammatory response, and cell death were evaluated.
Methods
Lungs and groups
Human donor lungs were obtained from a local organ procurement organization when the double lungs or right lungs were rejected by the clinical LTx team and used only for experiments. Family members gave consent for use of the donor lungs. Our selection criteria for research lungs were as follows: (I) uncontrolled infection; and/or (II) partial pressure of O2 in arterial blood/fraction of inhaled oxygen (P/F) <300 mmHg; and/or (III) abnormal chest X-ray/CT scan suggesting a localized lesion or atelectasis. The harvest procedure was performed in a standard fashion. The right donor lungs were preserved in cold low potassium dextran solution at 4 °C. To make the experimental results more obvious, we extended the normal cold storage time from 4–6 to 18–24 hours. After the cold storage, lungs were randomly assigned to two groups: the control group and the perfusate purification (PP) group.
EVLP
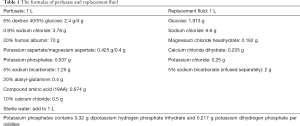
The circuit was primed with 2.0 L of the self-made perfusate (Table 1). The centrifugal pump circulated the perfusate through the membrane gas exchanger and arterial filter before entering the organ chamber. In the PP group, a dialyzer (FX 80, Fresenius, Germany) was added in to the circuit as a bypass (Figure 1). The replacement fluid (Table 1) mixed with sodium bicarbonate was reinfused to the circulation. The heater and gas tank containing a mixture of carbon dioxide (8%)/oxygen (6%)/nitrogen (84%) were connected to the membrane gas exchanger. Continuous assessment of the pulmonary arterial pressure (PAP), peak airway pressure (PAWP), and perfusate temperature were performed by the monitoring system.
Full table
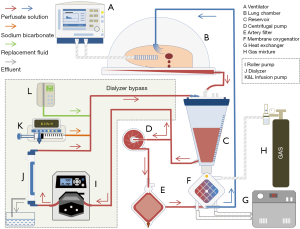
The right lungs were prepared and connected to the EVLP device, with the veins left open. After de-airing of the circuit, the lungs were slowly rewarmed to 37 °C over a 40 min period, with pulmonary flow allowed to increase gradually to 800 mL/h. Ventilation and PP were initiated at 32 °C. A protective mode of mechanical ventilation was applied with a tidal volume (Vt) of 4 mL/kg at 7 beats/min, positive end-expiratory pressure of 5 cmH2O and fraction of inspired oxygen (FIO2) of 0.21. In the PP group, the roller pump was adjusted according to the filtration speed maintained at 400 mL/h. The replacement fluid was infused at a dynamical adjusted speed to maintain the liquid level in the reservoir. In control group, the perfusate was exchanged for 250 mL fresh solution every hour.
Evaluation of the lungs was done every hour, continuously adjusting to physiologic metabolic conditions by adding glucose as necessary in the control group. During the evaluative phase, Vt increased to 6 mL/kg, FIO2 increased to 1.00, and respiratory rate increased to 10 beats/min for 5 min, while maintaining a PAWP limit of 25 cmH2O. Perfusate samples were taken from the pulmonary vein and artery hourly just at the end of evaluative phase.
EVLP termination
Preservation of the donor lungs in the EVLP was performed for 12 hours. Premature termination was considered in the following cases: (I) a large amount of edema fluid or foam visible in the airway; or (II) ΔPaO2 dropping below 300 mmHg in two consecutive tests (ΔPaO2 was equal to the difference between PaO2 of pulmonary vein and artery). After removing the lungs from the circulation, lung tissue samples were taken from the middle of the upper and lower lobes for pathological assessment.
Gas, electrolyte, and lactate analysis
Perfusate samples were taken for gas, electrolyte, and lactate analysis, which were measured using the CG8+ Test Cartridges and CG4+ Test Cartridges with i-STAT handheld (Abbott, Princeton, NJ, USA).
Inflammatory marker analysis
Cytokine profiles (TNF-α, IL-1β, IL-6, IL-8, and MCP-1) were analyzed using enzyme-linked immunosorbent assay kits (Multi Sciences, Hangzhou, China).
Histological and apoptosis assessment
The lung tissues fixed in 10% buffered formalin were embedded in paraffin and sectioned onto slides. The slides were stained with hematoxylin and eosin (HE), and evaluated with lung injury scores in a blinded manner.
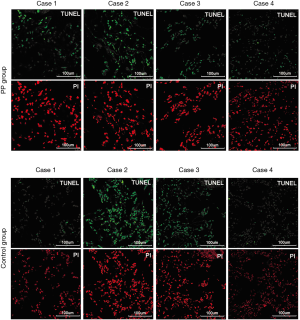
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining was performed to assess lung tissue apoptosis after EVLP using the one-step TUNEL apoptosis assay kit (Beyotime, Shanghai, China). Using a confocal laser scanning microscopy, 5 fields per slide were photographed. TUNEL positive green cells and red stained nuclei were counted in a blinded manner. The results were represented as the ratio of TUNEL positive cells to total cells.
Statistics
All results are expressed as mean ± SD and all statistical analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL, USA). The Student’s t-test was used to compare normally distributed continuous variables, chi-squared test was used for categorical variables, and two-way repeated measures ANOVA was used for significance within groups, over time. Statistical significance was measured at P<0.05.
Results
Donor lung and EVLP characteristics
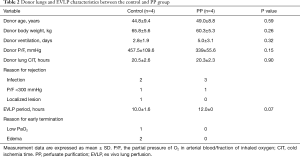
A total of 8 lungs were obtained and randomly divided into two groups, and each group had 4 cases of EVLP. Donor and donor lung parameters showed no significant differences between PP and control groups (Table 2), even though the PP group had a worse donor ventilation time and P/F. In the control group, only 1 case of EVLP was maintained for 12 h, 2 cases were terminated at the 8th and 11th hour due to severe edema, and 1 case was terminated at the 9th hour due to ΔPaO2 reduction to less than 300 mmHg. In the PP group, all the 4 cases were terminated conditionally after 12 h, and only 1 case showed a decrease of PaO2 after 11 h.
Full table
Donor lung physiology during EVLP
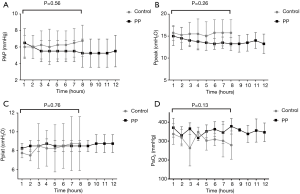
Donor lung physiology was monitored hourly throughout the 12 h of EVLP. PAP gradually increased after 6 h of EVLP in the control group, while it remained stable in the PP group (Figure 2A). The peak and plateau airway pressures were stable during the EVLP in both groups, and there were no significant differences between the two groups in the first 8 h (Figure 2B,C). ΔPaO2 was equal to the difference between the PaO2 of the pulmonary vein and artery, which was gradually decreased in the control group after 6 h of EVLP, but remained at the same levels in the PP group; however, there was still no significant difference between groups (Figure 2D).
pH, electrolyte, and lactate analysis
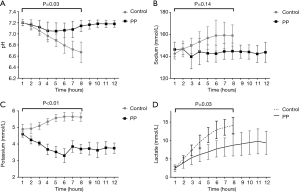
pH, electrolyte, and lactate analysis were examined hourly. pH was stable in the PP group but decreased markedly in the control group (Figure 3A). The same results also occurred in relation to the concentration of sodium and potassium (Figure 3B,C). Lactate was increased in both groups, but the increase rate in the PP group was significantly lower than that in the control group (Figure 3D). There were significant differences in the change trends of pH, potassium, and lactate between the two groups.
Inflammatory markers
Cytokine profiles (TNF-α, IL-1β, IL-6, IL-8, IL-10, and MCP-1) were analyzed at time points of 1, 3, 6, 9, and 12 h (Figure 4A,B,C,D,E,F). Each case showed a significant increase in IL-1β, IL-6, IL-8, IL-10, and MCP-1. TNF-α showed a change that rose at first, fell, and finally rose again. There was no significant difference between the two groups in TNF-α, IL-1β, IL-8, and MCP-1 levels. IL-6 (P=0.08) and IL-10 (P=0.01) were higher in the PP group in the first 6 h.

Histological and apoptosis assessment
Lung histology of both groups showed lung injury and no significant difference was noted in HE-stained slides. Cell apoptosis was examined by TUNEL staining and quantified in a blinded fashion. TUNEL-positive green cells and red-stained nuclei were counted (Figure 5). TUNEL positive cells in the control group (69.5%±4.0%) were significantly higher than in the PP group (47.5%±3.9%) (P=0.000).
Discussion
In clinic, the cold storage time is generally less than 12 h, and the average duration of EVLP is 4–6 h (5). To explore the method of replacing perfusate by adding perfusate filter device, we adopted the extreme condition in which EVLP was running for 12 h after being stored in 4 °C for 18–24 h. Furthermore, we invested in a new method of EVLP, which could prolong the running time under the extreme conditions. In our study, the control group maintained for an average of 10 h in accordance with the classical normothermic EVLP method, with 3 cases having premature termination of EVLP after reaching the terminate state. However, the physiological lung state of the PP group was stably maintained, while the TUNEL showed a lower cell apoptosis, even if this group was preserved longer. The significance of this lies not only in prolonging the time of EVLP, but also in reducing the use of perfusion fluid, without increasing the complexity of operation.
The application of EVLP is undoubtedly the most remarkable achievement in the field of LTx in the past decade. In 2012, Toronto General Hospital reported (6) 50 lung transplants being performed with marginal donor lungs assessed by EVLP, which accounted for about 20% of lung transplants in that period. According to a recent report (7), more than 460 lung transplants from EVLP donors have been performed in Toronto. A non-inferiority, randomized, controlled, open-label, phase 3 trial (INSPIRE) (8) was performed in the United States between 2011 to 2014, with 151 cases receiving donor lungs preserved with the OCS. The OCS Lung device significantly reduced the incidence of grade 3 primary graft dysfunction within 72 h after LTx.
The assessment of donor lung quality during EVLP mainly relies on the monitoring of airway pressure, pulmonary artery pressure, and oxygen concentration (9). Our study showed that in the control group, Ppeak and PAP were higher than those in the PP group, while P/F was lower in the PP group, but there was no statistical difference in this trend. It was shown that evaluating the lungs with P/F only did not adequately represent donor quality when using a cellular perfusion protocol (10). The P/F of the donor lung remained normal for a period of time even when there was obvious pulmonary edema. In addition, lung compliance could not be accurately obtained in the present study due to a unilateral lung being used in the experiment, so it was not analyzed. In order to minimize lung injury, the pulmonary artery flow was set at low flow to reduce pulmonary artery pressure in both groups.
In EVLP operation, regular monitoring electrolyte levels are necessary. In classical EVLP protocol, due to the absence of liver and kidney, the circulating concentration of potassium, sodium, and lactate will increase significantly, while the pH will continue to decline. Homeostasis disturbances and the accumulation of lactate, which have been discovered to play an immunosuppressive role in sepsis (11), may promote lung injury. In our study, the dialyzer in this experiment cleared lactate in circulation effectively, which may be the mechanism by which the apoptosis was reduced in the PP group.
Cytokines and chemokines are small molecules released during ischemia reperfusion (IR), and the lungs in EVLP go through a similar process (12). In a previous study (13), IL-1β, IL-1ra, IL-6, IL-8, IL-10, IL-12, and IL-18 continuously accumulated in perfusate throughout the perfusions, but adding a filter for clearance of inflammatory mediators improved EVLP physiology during the 12-hour perfusion period. In our study, the inflammatory cytokines increased significantly in both groups, but the differences were not significant between the two groups. These results indicate that our approach stabilizes the internal environment rather than clearing the inflammatory cytokines.
Another problem of EVLP is its high running cost. According to the DEVELOP-UK study (14), although EVLP increased the number of donor lungs available for transplant, it increased the cost of LTx (15). As the elapsed time of EVLP is extended, the continuous updating of perfusion fluid is required, and the cost will increase further. At least 8 liters of perfusate is required for a 24-hour long EVLP. In our modified method, only 2 L of perfusion solution is required, which significantly reduces the cost of EVLP.
Our study had some limitations. Lung function was assessed during EVLP, but no further LTx was performed. It is not clear what the effect of eliminating small molecular proteins and other substances by adding the dialyzer was, or which of them were protective factors and which were risk factors. Twenty-four-hour EVLP (16,17) had been reported only in healthy porcine lungs without ventilator-induced lung injury and systemic inflammation; but we can attempt a longer human lung EVLP by using our new method. In future studies, the best filtration rate should be evaluated, and serum and red blood cells can be added to simulate the environment in the body in order to achieve a real long-term preservation of lungs for several days.
Conclusions
Using the modified method of EVLP reduces the high cost caused by exchanging perfusion fluid per hour and prolonging the normothermic preservation time of donor lungs. The mechanism may be related to the reduction of lactate accumulation, the improvement of tissue environment, and reduced apoptosis.
Acknowledgments
Funding: The study is supported by the Jiangsu Eminent Medical Talents Project (JCRCB2016003), the Wuxi Clinical Medicine Center Project (LCZX001), and the Wuxi Science and Technology Development Fund (No. 2018125). The albumin used in the perfusate was provided by the Shanghai RAAS Blood Products Co., Ltd.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experiments were approved by the Ethics Committee of the Nanjing Medical University (NJMU-EA-2019-420). The donation after citizen’s death proceeded in accordance with the standardized process, and the consent of family members for research purpose was acquired.
References
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Inci I, Yamada Y, Hillinger S, et al. Successful lung transplantation after donor lung reconditioning with urokinase in ex vivo lung perfusion system. Ann Thorac Surg 2014;98:1837-8. [Crossref] [PubMed]
- Galasso M, Feld JJ, Watanabe Y, et al. Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion. Nat Commun 2019;10:481. [Crossref] [PubMed]
- Possoz J, Neyrinck A, Van Raemdonck D. Ex vivo lung perfusion prior to transplantation: an overview of current clinical practice worldwide. J Thorac Dis 2019;11:1635-50. [Crossref] [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [Crossref] [PubMed]
- Dong W. Prof. Shaf Keshavjee: innovating lung transplantation and facing the challenges of the unknown. J Thorac Dis 2019;11:E14-6. [Crossref] [PubMed]
- Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357-67. [Crossref] [PubMed]
- Okamoto T, Wheeler D, Liu Q, et al. Correlation between PaO(2)/FiO(2) and airway and vascular parameters in the assessment of cellular ex vivo lung perfusion system. J Heart Lung Transplant 2016;35:1330-6. [Crossref] [PubMed]
- Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. [Crossref] [PubMed]
- Nolt B, Tu F, Wang X, et al. Lactate and Immunosuppression in Sepsis. Shock 2018;49:120-5. [Crossref] [PubMed]
- Sadaria MR, Smith PD, Fullerton DA, et al. Cytokine expression profile in human lungs undergoing normothermic ex-vivo lung perfusion. Ann Thorac Surg 2011;92:478-84. [Crossref] [PubMed]
- Iskender I, Cosgun T, Arni S, et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J Heart Lung Transplant 2017. [Epub ahead of print]. [PubMed]
- Fisher A, Andreasson A, Chrysos A, et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1-276. [Crossref] [PubMed]
- McMeekin N, Chrysos AE, Vale L, et al. Incorporating ex-vivo lung perfusion into the UK adult lung transplant service: an economic evaluation and decision analytic model. BMC Health Serv Res 2019;19:326. [Crossref] [PubMed]
- Sommer W, Salman J, Avsar M, et al. Prediction of transplant outcome after 24-hour ex vivo lung perfusion using the Organ Care System in a porcine lung transplantation model. Am J Transplant 2019;19:345-55. [Crossref] [PubMed]
- Spratt JR, Mattison LM, Iaizzo PA, et al. Lung transplant after prolonged ex vivo lung perfusion: predictors of allograft function in swine. Transpl Int 2018;31:1405-17. [Crossref] [PubMed]