
Efficacy and safety of Sijunzi Decoction for chronic fatigue syndrome with spleen deficiency pattern: study protocol for a randomized, double-blind, placebo-controlled trial
Introduction
Chronic fatigue syndrome (CFS) is characterized by prolonged and disabling fatigue (>6 months), as well other nonspecific somatic symptoms (1). Epidemiological information of CFS is limited. Available studies reported the prevalence of CFS fluctuated from 0.23% to 2.6% (2-4). Although not life-threatening, CFS would severely impact patients’ quality of life and lead to tremendous social burden (5,6).
Due to the unclear understanding of CFS pathophysiology, current interventions focus on symptomatic treatment and psychological behavior therapies (7). Based on current trials, cognitive behavioral therapy and graded exercise therapy possess relatively high-quality evidences. However, due to the conventional doctor-visiting custom and psychological barrier in Chinese population (8,9), these two therapies cannot get comprehensive application. In addition, existed trials related to chemical agents did not get satisfactory results (10-13). Therefore, many Chinese patients would seek help from traditional Chinese medicine (TCM), in which Chinese herbal medicine (CHM) is the main form.
According to the TCM concept, spleen governs energy metabolism. Spleen deficiency would lead to reduced energy, which is a manifestation of CFS (14). Therefore, we choose “spleen deficiency” as the accompanying TCM pattern. Sijunzi Decoction (SJZD), which was served as the basic formula treating spleen deficiency, has been utilized for fatigue since Tang Dynasty (15). The four ingredients, namely Renshen (Radix Ginseng), Baizhu (Rhizoma Atractylodis Macrocephalae), Fulin (Poria) and Zhigancao (Radix Glycyrrhizae Preparata), showed certain pharmacological effects in energy metabolism, such as increasing adenosine triphosphate (ATP) level, regulating activity of glucokinase and phosphoglycerate kinase, regulating lipid synthesis, and stimulating mitochondrial function (16-21). However, there still lack of high-quality clinical evidence related to its application. Hence, we design this rigorous randomized, double-blind, clinical trial, to evaluate the actual effectiveness and safety of SJZD using placebo as comparator, and thus provide preliminary support for its application in CFS management.
Methods and design
Study objectives
This clinical trial aims to assess the efficacy and safety of SJZD for CFS patients.
Study design and setting
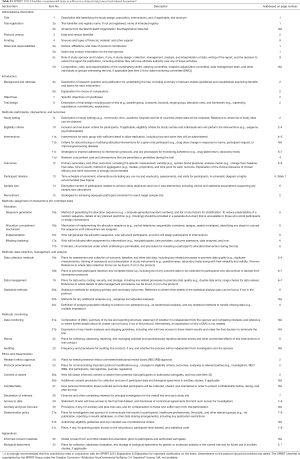
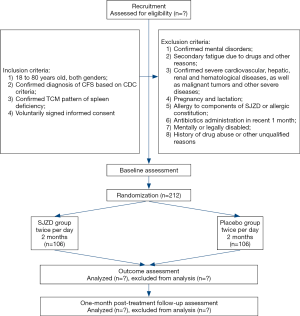
This trial is designed as a single-center, double-blind, placebo-controlled trial with two parallel groups to evaluate the effect of SJZD. The protocol was developed based on Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement and TCM extension (22-24). The SPIRIT checklist is presented in Supplementary (Table S1). A total of 212 CFS patients will be recruited. After inform consent acquisition and eligibility confirmation, participants will be randomly allocated to SJZD group and placebo group based on the ratio of 1:1. On the foundation of general health education, participants will go through a 2-month treatment period receiving either SJZD or placebo, and then a 1-month follow-up period. The participant flowchart is shown in Figure 1, and the participant timeline is given in Table 1.
Full table
Full table
Four visits will be arranged for each participant, namely baseline (visit 1), 1 month (visit 2), 2 months (visit 3) and 3 months (visit 4). At visit 1, clinical investigator will confirm participant’s qualification, document general information and then give the first-month medications. Participants will be required to come back at visit 2 for midterm clinical evaluation and second-month medications. Visit 3 is set for the end of the treatment period, and only clinical evaluation will be arranged. Afterwards, visit 4 is set for evaluating the continued effect.
The study protocol (version: PZYH-DL-1.1) was approved by Medical Ethics Committee of Longhua Hospital Affiliated to Shanghai University of TCM (approval number: 2019LCSY020), and was registered in the ISRCTN registry (ISRCTN23930966). If there will be any changes in study design, Ethics Committee would be informed immediately. Informed consent will be obtained from each patient.
Participants
This clinical trial will be conducted at the Longhua Hospital Affiliated to Shanghai University of TCM. All participants will be recruited from the public through the outpatient clinic.
Diagnostic criteria
The diagnosis of CFS will be confirmed based on the Centers for Disease Control and Prevention (CDC) criteria (1). To be specific, individuals should have severe fatigue for longer than 6 months, and possess at least four of the following symptoms: (I) headache of new type, pattern, or severity; (II) multijoint pain without swelling or erythema; (III) muscle pain; (IV) postexertional malaise for longer than 24 hours; (V) significant impairment in short-term memory or concentration; (VI) sore throat; (VII) tender lymph nodes; (VIII) unrefreshing sleep.
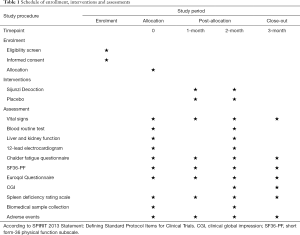
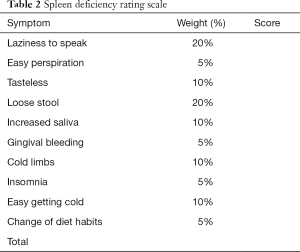
The pattern differentiation criteria for spleen deficiency refers to previous study and related guidelines (25-27). In brief, ten symptoms will be evaluated by patients themselves using 0–100 grading scale, namely (I) laziness to speak; (II) easy perspiration; (III) tasteless; (IV) loose stool; (V) increased saliva; (VI) gingival bleeding; (VII) cold limbs; (VIII) insomnia; (IX) easy getting cold; (X) change of diet habits. Higher scoring indicates higher severity. Each symptom has its own weighting factor. Clinical investigators with more than 5-year experience will be responsible for scale inquiry. Spleen deficiency is defined as the accumulative scores of all symptoms above 20. The detailed rating scale is shown in Table 2.
Full table
Inclusion criteria
Participants who meet all of the following criteria could be included: (I) 18 to 80 years old, both genders; (II) confirmed diagnosis of CFS based on CDC criteria; (III) confirmed TCM pattern of spleen deficiency; (IV) voluntarily signed informed consent.
Exclusion criteria
Participants who meet any of the following criteria will be excluded: (I) confirmed mental disorders; (II) secondary fatigue due to drugs and other reasons; (III) confirmed severe cardiovascular, hepatic, renal and hematological diseases, as well malignant tumors and other severe diseases; (IV) pregnancy and lactation; (V) allergy to components of SJZD or allergic constitution; (VI) antibiotics administration in recent 1 month; (VII) mentally or legally disabled; (VIII) history of drug abuse or other unqualified reasons.
Interventions
General medical education
During every visit, clinical investigators will inform participants in either group essential medical education information, such as taking breaks timely, regular physical exercise, keeping smooth state, and avoiding negative emotions. Symptomatic treatment for accompanying symptoms will also be allowed.
CHM intervention
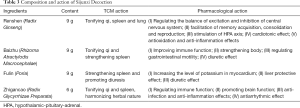
SJZD is composed of Renshen (Radix Ginseng), Baizhu (Rhizoma Atractylodis Macrocephalae), Fulin (Poria) and Zhigancao (Radix Glycyrrhizae Preparata). The specific composition of one daily dosage and related actions are listed in Table 3. The placebo is the simulant granule, which the main components are starch and dextrin. In order to present comparable color, smell, taste and texture with SJZD, food colorants and flavoring agents are added. Both SJZD and placebo are prepared as soluble granule by a third company, which is qualified in CHM processing and preparation. The third pharmaceutical company will equally divide daily dose (SJZD or placebo) into two individual pack. Participants from either group will be required to dissolve one pack with 200 mL hot water and take the decoction orally twice daily for 2 months. Both SJZD and placebo will be packed in sealed medicine box. Only the serial number will be printed outside the package to ensure successful blinding.
Full table
Outcomes
Throughout the study, participants will be required to complete a series of questionnaires. Due to the unclear pathophysiology mechanism of CFS, no efficacy related biochemical test will be arranged. However, blood sample and stool sample will still be collected at baseline and treatment endpoint. for future deep mechanism exploration. At baseline, clinical investigators will obtain inform consent and document general information such as age, gender, height, weight and medical history. For safety concern, routine blood test, liver and kidney function test and 12-lead electrocardiogram (ECG) will be conducted at baseline and treatment endpoint (2 months). Additionally, vital signs and adverse events (AEs) will be monitored during the whole study.
The primary outcome will be the change of Chalder fatigue questionnaire after treatment. The Chalder fatigue questionnaire, which contains 11 questions in total, is a classic questionnaire measuring fatigue and has been widely used in previous CFS clinical trials (28-30). Each question includes four options, namely “better than usual”, “no more than usual”, “worse than usual” and “much worse than usual”, indicating Likert scoring 0, 1, 2 and 3, respectively. The cumulative score ranges from 0 to 33, and lowest score is least fatigue.
Secondary outcomes include the short form-36 physical function subscale (SF36-PF), spleen deficiency rating scale, quality of life [assessed by the Euroqol Questionnaire (EQ-5D-5L)] and self-rated clinical global impression scales [including overall health (CGI-health) and CFS (CGI-CFS)]. SF36-PF is a valid and commonly-used assessment tool in evaluating the impact of CFS on patient’s daily life (29,30). The score ranges from 0 to 100, and the highest score indicates best function. In this trial, we introduce the concept of TCM spleen deficiency, thus the spleen deficiency rating scale will be used for quantificationally assessing the change of TCM pattern. EQ-5D-5L is a modified measure of quality of life, and has also been applied in related studies (30-33). It contains an EQ-5D descriptive system focusing five dimensions, and an EQ-Visual Analogue Scale (VAS) scoring the overall health status. CGI scale contains seven options, which could be categorized into three classes: negative change (very much worse or much worse), no obvious change (a little worse, no change, or a little better), and positive change (much better or very much better). This is a self-rated tool assisting participant to recognize the therapeutic change from baseline (30).
Safety outcomes include documentation of AEs, physical examination, vital signs, and the relevant laboratory examination mentioned above. If any AEs happen, clinical investigators should record the onset time, related symptoms and signs, duration, abnormal laboratory indexes, intervention and prognosis. Then, the relationship with experimental drugs will be evaluated based on these data. The detailed arrangements of every outcome are presented in Table 1.
Randomization and blinding
Eligible participants will be randomly and equally allocated in two SJZD group or placebo group based on randomization sequence table generated by SPSS 22.0 for Windows. A specific statistical researcher who does not participate in the clinical trial will be responsible for generating the randomization sequence and distribute the number to the experimental products. Afterwards, clinical investigators will randomly assign the drug based on enrollment order. Intentionally selecting is strictly forbidden. The blinding base containing the randomization sequence, parameters of sequence, and treatments assignment is sealed and reserved by the principal investigator. Participants and clinical investigators will only notice the individual random number. At the last visit, participants and clinical investigators will complete a questionnaire about treatments assignment to verify the success of blinding. Emergency letters containing random number and treatment assignment will also be prepared by the specific statistical researcher. Only emergency when the actual intervention is necessary for further management could allow code breaks.
Sample size calculation
The sample size estimation is based on the primary outcome. To our knowledge, this is the first clinical trial assessing effect of a CHM formula on CFS with a specific TCM pattern, no previous related data could be found. Therefore, after discussion with clinical specialists, we assume that SJZD could reduce 3 point in Chalder fatigue scale, while placebo could reduce 0.5 point. In addition, based on previous study, the CFS patients had a mean fatigue score of 24.4±5.8 (34). Therefore, the sample size of each group could be calculated by using the following formula (35):
We set type I error α=0.05 and a power of 90% (β=0.10). Two equal groups are designed, hence k=1. As mentioned above, σ will be 5.8, and δ will be 2.5. Ninety-two subjects are needed in one single group. Considering a 15% drop-out, a total of 212 subjects are determined.
Statistical analysis
All outcomes will be analyzed based on the intention-to-treat (ITT) principal. The missing value will be filled up by last-observation-carried-forward method. SPSS 22.0 for Windows will be used, and the statistical significance is defined as two-tailed P<0.05. For the quantitative data, mean, standard deviation (SD), minimum, maximum and median will be reported. The paired quantitative data will also present the mean and SD of difference. For the enumerative data, frequency and corresponding percentage will be given. For intra-group comparisons during the study, paired t test or Wilcoxon signed rank test will be used for quantitative indexes, and chi-squared test will be utilized for qualitative indexes. For comparisons between groups, two-sample t-test or suitable non-parameter methods will be applied for quantitative indexes, and chi-squared test will be utilized for qualitative indexes. Clinical effectiveness should be confirmed after evaluating the clinical significance.
Data collection and quality control
This is a 3-month clinical trial. During the whole study, participants will be required to take assigned medication for consecutive 2 months, visit clinical investigator at four timepoints, complete some questionnaires and provide two rounds biomedical samples. The original data will be recorded comprehensively in case report form (CRF). Nobody except for principal investigators and clinical investigators is qualified to review the documents. The original records will be preserved at least 5 years after study completion.
In order to enhance the quality and stability of this clinical trial, clinicians with more than 5-year experience are eligible to be clinical investigators. Before the recruitment, all related researchers will undergo several training courses to guarantee the comprehensive understanding of study protocol, questionnaire evaluation and research process. Participants’ compliance is a vital factor of the success of clinical trial. Hence, the following principles will be executed throughout the study. Firstly, clinical investigators should strictly comply with the principle of informed consent and assist participants to understand the possible benefits and risks. Secondly, clinical investigators should require participants to bring the used packs back in visit 2 and 3, then to examine the administration situation. Thirdly, investigators may contact participants through phone and texts to remind the following visit three days in advance. For participants who present poor compliance, investigators should ask possible reasons and encourage them to complete the study.
Discussion
CFS has become a widespread problem worldwide. However, the underlying etiology of CFS is still in the mist. Early researches reported that post viral infections, such as Epstein-Barr virus (EBV), enterovirus and xenotropic murine leukemia virus-related virus (XMRV), could be noticed in CFS patients (36-38). However, the evidences are not consolidated (39,40). Abnormal immune function is also a potential etiological factor of CFS, due to the findings which CFS patients manifested imbalanced natural killer (NK) cells and interleukin (IL) (41,42). Similarly, some researches also showed conflicted results (43,44). In addition, CFS patients may present lower cortisol level (45), certain genetic susceptibility (46), and negative psychological mood (47). Hence, based on available researches, the onset of CFS may be related to immune system, neuroendocrine system, genetics and biopsychosocial model.
To our knowledge, this is the first randomized controlled clinical trial evaluating a CHM formula for CFS treatment. SJZD is a classical prescription in clinical practice, and for the underlying pathogenesis mentioned above, a variety of pharmacological studies have illustrated the potential suitability of SJZD in CFS management. For instance, recent study showed that modified SJZD could regulate immune disorders in chronic atrophic gastritis patients with fatigue and tiredness symptom (48). And in a special immunosuppression rat model, SJZD could also improve immune function by regulate janus kinase (JAK)-signal transducer and activator of transcription (STAT) signal pathway (49). Besides, in over fatigue and irregular diet rat model, SJZD could improve hypothalamic-pituitary-adrenal (HPA) axis function by elevating the level of adrenocorticotropic hormone (ACTH) and corticosterone (50). Therefore, on the foundation of previous researches, it is reasonable to choose SJZD as the intervention.
In this clinical trial, we choose generally-accepted scale as our outcomes. Nevertheless, we will still collect participants’ blood and stool samples for potential mechanism exploration. Gut microbiota is considered to be an important site of energy metabolism (51). Hence, we curiously wonder whether the effect brought by SJZD, if any, involves the alteration of gut microbiota. This is also an innovative attempt among similar type trials.
In conclusion, this protocol is strictly developed according to the requirements of SPIRIT statement and corresponding extension. The results generated from this rigorous design would have high reliability, and provide preliminary evidence of SJZD in CFS management.
Acknowledgments
Funding: This work is supported by Shanghai Three-year Action Plan for Accelerating the Development of Traditional Chinese Medicine [ZY(2018-2020)-CCCX-2002-01].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by Medical Ethics Committee of Longhua Hospital Affiliated to Shanghai University of TCM (Approval Number: 2019LCSY020). Informed consent will be obtained from each patient.
References
- Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994;121:953-9. [Crossref] [PubMed]
- Wessely S, Chalder T, Hirsch S, et al. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health 1997;87:1449-55. [Crossref] [PubMed]
- Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med 1999;159:2129-37. [Crossref] [PubMed]
- Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med 2003;163:1530-6. [Crossref] [PubMed]
- Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med (Lond) 2005;55:20-31. [Crossref] [PubMed]
- Reynolds KJ, Vernon SD, Bouchery E, et al. The economic impact of chronic fatigue syndrome. Cost Eff Resour Alloc 2004;2:4. [Crossref] [PubMed]
- Cleare AJ, Reid S, Chalder T, et al. Chronic fatigue syndrome. BMJ Clin Evid 2015;2015.
- Chen J. Some People May Need it, But Not Me, Not Now: Seeking Professional Help for Mental Health Problems in Urban China. Transcult Psychiatry 2018;55:754-74. [Crossref] [PubMed]
- Sun KS, Lam TP, Lam KF, et al. Barriers and facilitators for psychiatrists in managing mental health patients in Hong Kong-Impact of Chinese culture and health system. Asia Pac Psychiatry 2018. [Crossref] [PubMed]
- Cleare AJ, Miell J, Heap E, et al. Hypothalamo-pituitary-adrenal axis dysfunction in chronic fatigue syndrome, and the effects of low-dose hydrocortisone therapy. J Clin Endocrinol Metab 2001;86:3545-54. [Crossref] [PubMed]
- Blockmans D, Persoons P, Van Houdenhove B, et al. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: a randomized, placebo-controlled, double-blind, crossover study. Am J Med 2003;114:736-41. [Crossref] [PubMed]
- Kogelnik AM, Loomis K, Hoegh-Petersen M, et al. Use of valganciclovir in patients with elevated antibody titers against Human Herpesvirus-6 (HHV-6) and Epstein-Barr Virus (EBV) who were experiencing central nervous system dysfunction including long-standing fatigue. J Clin Virol 2006;37 Suppl 1:S33-8. [Crossref] [PubMed]
- Chambers D, Bagnall AM, Hempel S, et al. Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. J R Soc Med 2006;99:506-20. [PubMed]
- Deng T, Guo Z. Diagnosis in Chinese Medicine. Shanghai: Shanghai Science and Technology Press, 1984.
- Xu J, Wang M. Formulas of Chinese Medicine. Shanghai: Shanghai Science and Technology Press, 1984.
- Li XT, Chen R, Jin LM, et al. Regulation on energy metabolism and protection on mitochondria of Panax ginseng polysaccharide. Am J Chin Med 2009;37:1139-52. [Crossref] [PubMed]
- Wang JR, Zhou H, Yi XQ, et al. Total ginsenosides of Radix Ginseng modulates tricarboxylic acid cycle protein expression to enhance cardiac energy metabolism in ischemic rat heart tissues. Molecules 2012;17:12746-57. [Crossref] [PubMed]
- Song MY, Kang SY, Oh TW, et al. The Roots of Atractylodes macrocephala Koidzumi Enhanced Glucose and Lipid Metabolism in C2C12 Myotubes via Mitochondrial Regulation. Evid Based Complement Alternat Med 2015;2015:643654.
- Song MY, Lim SK, Wang JH, et al. The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. Int J Mol Sci 2018. [Crossref] [PubMed]
- Han XY, Wang YN, Dou DQ. Regulatory effects of Poria on substance and energy metabolism in cold-deficiency syndrome compared with heat-deficiency syndrome in rats. Chin J Nat Med 2018;16:936-45. [PubMed]
- Madak-Erdogan Z, Gong P, Zhao YC, et al. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Mol Nutr Food Res 2016;60:369-80. [Crossref] [PubMed]
- Dai L, Cheng CW, Tian R, et al. Standard Protocol Items for Clinical Trials with Traditional Chinese Medicine 2018: Recommendations, Explanation and Elaboration (SPIRIT-TCM Extension 2018). Chin J Integr Med 2019;25:71-9. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [Crossref] [PubMed]
- Zheng X. Guiding principles for clinical research of new TCM drugs (on trial). Beijing: China Medical Science Press, 2002.
- Lin J. Development and assessment of PRO scale of qi deficiency of spleen. Fujian University of Traditional Chinese Medicine, 2013.
- Zhang SS, Hu L, Li RL. Expert consensus on diagosis and treatment of spleen deficiency with Chinese Medicine. Journal of Traditional Chinese Medicine 2017;58:1525-30.
- Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res 1993;37:147-53. [Crossref] [PubMed]
- White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet 2011;377:823-36. [Crossref] [PubMed]
- Clark LV, Pesola F, Thomas JM, et al. Guided graded exercise self-help plus specialist medical care versus specialist medical care alone for chronic fatigue syndrome (GETSET): a pragmatic randomised controlled trial. Lancet 2017;390:363-73. [Crossref] [PubMed]
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. [Crossref] [PubMed]
- Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. [Crossref] [PubMed]
- Kim JE, Seo BK, Choi JB, et al. Acupuncture for chronic fatigue syndrome and idiopathic chronic fatigue: a multicenter, nonblinded, randomized controlled trial. Trials 2015;16:314. [Crossref] [PubMed]
- Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res 2010;69:17-22. [Crossref] [PubMed]
- Wang J. Clinical epidemiology_design, measure and appraise of clinical researches. 4th ed. Shanghai: Shanghai Science and Technology Press, 2014.
- Straus SE, Tosato G, Armstrong G, et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med 1985;102:7-16. [Crossref] [PubMed]
- Chia JK. The role of enterovirus in chronic fatigue syndrome. J Clin Pathol 2005;58:1126-32. [Crossref] [PubMed]
- Lombardi VC, Ruscetti FW, Das Gupta J, et al. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 2009;326:585-9. Erratum in: Science. 2011 Oct 14;334(6053):176. Retraction in: Alberts B. Science. 2011 Dec 23;334(6063):1636. [Crossref] [PubMed]
- Mawle AC, Nisenbaum R, Dobbins JG, et al. Seroepidemiology of chronic fatigue syndrome: a case-control study. Clin Infect Dis 1995;21:1386-9. [Crossref] [PubMed]
- van Kuppeveld FJ, de Jong AS, Lanke KH, et al. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ 2010;340:c1018. [Crossref] [PubMed]
- Brenu EW, Staines DR, Baskurt OK, et al. Immune and hemorheological changes in chronic fatigue syndrome. J Transl Med 2010;8:1. [Crossref] [PubMed]
- Fletcher MA, Zeng XR, Barnes Z, et al. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med 2009;7:96. [Crossref] [PubMed]
- Vernon SD, Reeves WC. Evaluation of autoantibodies to common and neuronal cell antigens in Chronic Fatigue Syndrome. J Autoimmune Dis 2005;2:5. [Crossref] [PubMed]
- Lorusso L, Mikhaylova SV, Capelli E, et al. Immunological aspects of chronic fatigue syndrome. Autoimmun Rev 2009;8:287-91. [Crossref] [PubMed]
- Cevik R, Gur A, Acar S, et al. Hypothalamic-pituitary-gonadal axis hormones and cortisol in both menstrual phases of women with chronic fatigue syndrome and effect of depressive mood on these hormones. BMC Musculoskelet Disord 2004;5:47. [Crossref] [PubMed]
- Whistler T, Jones JF, Unger ER, et al. Exercise responsive genes measured in peripheral blood of women with chronic fatigue syndrome and matched control subjects. BMC Physiol 2005;5:5. [Crossref] [PubMed]
- Chalder T, Godfrey E, Ridsdale L, et al. Predictors of outcome in a fatigued population in primary care following a randomized controlled trial. Psychol Med 2003;33:283-7. [Crossref] [PubMed]
- Tian G, Wu C, Li J, et al. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res 2019;144:158-66. [Crossref] [PubMed]
- Xiong B, Qian H. Effects of Sijunzi decoction and Yupingfeng powder on expression of janus kinase-signal transducer and activator of transcription signal pathway in the brain of spleen-deficiency model rats. J Tradit Chin Med 2013;33:78-84. [Crossref] [PubMed]
- Wang H. Comparison of biological effects of Sijunzitang (SJZT), Chaishusijuntang (CSSJT) and Chaihushugansan (CHSGS) on the animal model of the syndromes of spleen-qi deficiency [Medical Doctor]: Beijing University of Chinese Medicine, 2007.
- Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci 2016;73:737-55. [Crossref] [PubMed]


