Norepinephrine intravenous prophylactic bolus versus rescue bolus to prevent and treat maternal hypotension after combined spinal and epidural anesthesia during cesarean delivery: a sequential dose-finding study
Introduction
Norepinephrine, as a relatively new drug in obstetric anesthesia, has α-adrenergic and weak β-adrenergic effects. Compared with phenylephrine, it is less likely to induce bradycardia and decreased cardiac output (1-3), which makes it a potential alternative to phenylephrine.
Prophylactic use of vasopressors is increasingly considered to be a standard measure during spinal anesthesia (SA) for cesarean delivery (CD) (4-7). However, few developing countries incorporate prophylactic vasopressors into their delivery management guidelines (8).
This prospective, double-blind, randomized, sequential dose finding study aims to identify the 90% effective dose (ED90) value of norepinephrine for both prophylactic and rescue bolus doses by using the biased coin up-and-down (BCUD) design.
Methods
Materials and methods
After obtaining approval from the Research Ethics Committee of the International Peace Maternity and Child Health Hospital and registering at http://www.chictr.org.cn (INR-1800016213), we began a prospective, double-blind, randomized, sequential dose-finding study, using a BCUD design to estimate ED90. A total of 109 women aged 18–40 years, with a full-term (>37 weeks’ gestation) singleton pregnancy, ASA score II, and undergoing an elective CD were recruited during the study period from January 2018 to August 2018. After providing signed informed consent, the women were randomly allocated into two groups according to the randomized numbers generated by a research assistant using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA): the prophylactic bolus group (Group P) and the rescue bolus group (Group R). Exclusion criteria for the study were as follows: allergy to norepinephrine, preexisting or pregnancy-induced hypertension, preeclampsia, cardiovascular or cerebrovascular disease, fetal distress or fetal abnormalities, multiple gestations, patient refusal, and emergent CD.
Patients were instructed to fast after midnight on the day of surgery. After entering the operating room, an intravenous (IV) line was established with an 18-G IV catheter in the left forearm, and lactated Ringer’s solution was infused at a rate of 1 mL/min to maintain the vein open. Routine monitoring, including electrocardiography, non-invasive blood pressure, and pulse oximetry, was performed continuously. Systolic blood pressure (SBP), mean arterial pressure (MAP), heart rate (HR), and pulse oximetry were assessed once a minute (min). The first two resting SBP, MAP, and HR measurements in a supine position were recorded, and their average values were treated as the baseline pressure and HR. If the baseline SBP was above 140 mmHg, the patient was excluded from the study due to hypertension. The combined spinal and epidural (CSE) anesthesia was performed by experienced anesthesiologists. The CSE puncture was performed routinely at the level of L3–4 with the patient in the right lateral decubitus position. A 17G Tuohy needle was used to perform the epidural puncture with a paramedian approach. After identifying entrance into the epidural space, a 27-G Whitacre needle was then inserted through the epidural needle. As soon as the cerebrospinal fluid was detected, 10.5 mg (9) of 0.75% isobaric ropivacaine was injected through the Whitacre needle. After injecting the ropivacaine, an epidural catheter was inserted into the epidural space by advancing it 3 cm through the Tuohy needle. After the patient was moved to a supine position with left uterine displacement created by placing a wedge under the right hip, 5 mL of 2% lidocaine was administered through the epidural catheter. In addition, an infusion of 1 mL/kg/min lactated Ringer’s solution was administered until delivery. Another 5 mL of 2% lidocaine was administered through the epidural catheter 3 min after the initial dose if the patient could still feel the cold sensation of ice being placed on the skin below the level of T6. Patients who still had a sensory response to ice below the level of T6 at the beginning of surgery were excluded from the study. Immediately after delivery, 1 mL of umbilical vein (UV) blood was collected by the obstetrician, and blood gas assessments were performed using a blood gas analyzer (iSTAT1 Analyzer MN:300-G, Abbott Point of Care Inc., USA) with an iSTAT CG7+ test cartridge.
In Group P, a 5-mL prophylactic dose of norepinephrine in a 5-mL syringe prepared by a research assistant was administered via the IV catheter immediately after the patient was moved to a supine position. An additional 5-mL syringe with a rescue bolus of 6 µg of norepinephrine was prepared by the research assistant, and the patient received the rescue norepinephrine bolus of 6 µg immediately after the first episode of hypotension was detected. In Group R, 5 mL of normal saline in a 5-mL syringe prepared by the research assistant was administered immediately after the patient was moved to a supine position. Then, the rescue dose of norepinephrine in a 5-mL syringe prepared by the research assistant was administered immediately after the first episode of hypotension was detected. The research assistant was the only person who knew which drugs and doses were in which syringes. All anesthesiologists and patients were blinded to the drugs and doses. Hypotension was defined as having a SBP <80% of the baseline value. If the SBP of patients in either group could not be maintained at 80% of the baseline after the initial drug administration, then the additional rescue norepinephrine bolus of 6 µg in a 2-mL syringe would be administered by the anesthesiologist. To differentiate from the initial drugs, the additional rescue norepinephrine boluses of 6 µg were prepared in 2-mL syringe by anesthesiologist. If bradycardia, defined as HR below 50 beats per minute (BPM), was detected, then 0.5 mg atropine was administered to increase HR. The atropine and norepinephrine were given separately. Hypertension was defined as a SBP >120% of the baseline value.
The bolus dose of 4 µg was used for the first patient of both groups. The dose for subsequent patients was determined by the response of each previous patient. In Group P, if the SBP decreased below 80% of the baseline, the bolus dose was considered failed and the dose for the following patient was increased by 1 µg. If the SBP was maintained to at least 80% of the baseline, the dose used was considered a success, and the next patient was randomly assigned a dose with a 1/9th chance of receiving a lower dose (decreased by 1 µg) or an 8/9th chance of receiving the same dose as the previous patient. In Group R, if the SBP remained below 80% of the baseline for 2 successive 2 minutes blood pressure cycles after the bolus dose was given, or could be not maintained above 80% of the baseline until delivery, the dose was considered to have failed, and the dose for the following patient was increased by 1 µg. If the SBP was maintained above 80% of the baseline, the dose was considered to be a success and the next patient was randomly assigned a dose with a 1/9th chance of receiving a lower dose level (decreased by 1 µg) or an 8/9th chance of receiving the same dose level. This was implemented using the BCUD scheme prepared by a study statistician in Microsoft Excel 2016, and was used by the research assistant, who was the only person with access to this software, maintaining the double-blind nature of the study.
The primary outcome was the success of both the prophylactic bolus dose and the rescue bolus dose to maintain the SBP above 80% of the baseline until delivery. Secondary outcomes included both maternal and fetal observations. Maternal observations included dizziness, breathlessness, nausea (spontaneous complaints from patients recorded by anesthesia providers), vomiting, bradycardia requiring the use of atropine, use of additional rescue norepinephrine doses, hypertension, upper sensory level of anesthesia to ice cold, total co-load IV fluid, total consumption of norepinephrine. Neonatal observations included induction-delivery interval, uterine incision-delivery interval, UV blood gases [including pH, pO2, pCO2, base excess (BE), pHCO3, and SO2], neonatal weight, and Apgar scores measured at 1 and 5 min post-delivery. Maternal demographics such as age, weight, height, gestational weeks, gravida, para, baseline SBP, baseline MAP, and baseline HR were also recorded.
Statistical analysis and sample size calculation
This dose finding study based on BCUD design and simulation studies suggests that the stopping rule of enrolling 20–40 patients will provide stable estimates of the target dose for most realistic cases (10). In this study, the sample size of each group was 40 patients.
The ED90, defined as the bolus dose of norepinephrine at which the primary outcome of success was observed in 90% of the patients in the study population, was estimated by isotonic regression method (10,11). The isotonic regression estimator of ED90 is the linear interpolated dose between p*(r) and p*(r+1): 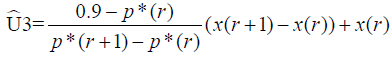 , where x(r)=max(x(i): p*(i)≤0.9) and p*(i) is the adjusted rate of the primary outcome of success at dose x(i), i=1, 2, 3,…, k, estimated by the pooled-adjacent-violators algorithm (PAVA). Because the observed rate of p={p(1), p(2),…, p(k)} might not be increased with respect to the dose level, which is the implicit assumption of a dose-finding study, the PAVA algorithm was first used in isotonic regression to obtain an increase adjusted rate p*={p*(1) ≤ p*(2) ≤, …, ≤ p(k)} based on p. The 95% confidence interval (CI) of isotonic regression estimator of ED90 was obtained by a bias-corrected percentile method (12,13) using 2,000 bootstrap replications of
, where x(r)=max(x(i): p*(i)≤0.9) and p*(i) is the adjusted rate of the primary outcome of success at dose x(i), i=1, 2, 3,…, k, estimated by the pooled-adjacent-violators algorithm (PAVA). Because the observed rate of p={p(1), p(2),…, p(k)} might not be increased with respect to the dose level, which is the implicit assumption of a dose-finding study, the PAVA algorithm was first used in isotonic regression to obtain an increase adjusted rate p*={p*(1) ≤ p*(2) ≤, …, ≤ p(k)} based on p. The 95% confidence interval (CI) of isotonic regression estimator of ED90 was obtained by a bias-corrected percentile method (12,13) using 2,000 bootstrap replications of 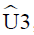 . Each replication was obtained by drawing a bootstrap data set with sample size of 40 and BCUD design, assuming that the true dose-response rate at each dose is p*(i), i=1, 2, 3, …, k, estimated based on the original data. We then estimated
. Each replication was obtained by drawing a bootstrap data set with sample size of 40 and BCUD design, assuming that the true dose-response rate at each dose is p*(i), i=1, 2, 3, …, k, estimated based on the original data. We then estimated 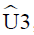 , the isotonic regression estimator of ED90 based on the bootstrap data. The isotonic regression and bootstrapping were performed by the study statistician using R version 3.4.4.
, the isotonic regression estimator of ED90 based on the bootstrap data. The isotonic regression and bootstrapping were performed by the study statistician using R version 3.4.4.
The data composed of demographic variables and secondary outcomes were summarized as mean ± standard deviations (SD), median, range, numbers, and proportions. Parametric data were analyzed with the t-test and nonparametric data were analyzed with the Mann-Whitney test. Comparison of proportions was performed using Chi-square test and Fisher exact tests as appropriate. Statistical comparisons were made using SPSS for Windows version 18.0 (SPSS Inc., IL, USA). Statistical significance was assumed if P<0.05.
Results
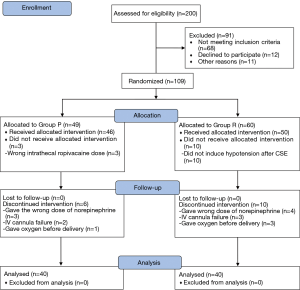
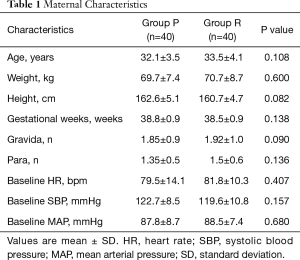
Two hundred women who underwent elective CSE for CD were selected for the study during January 2018 to August 2018. Data used for patient recruitment are shown in the Figure 1. Forty patients from each group were included in final data analysis. Maternal characteristics are shown in Table 1.
Full table
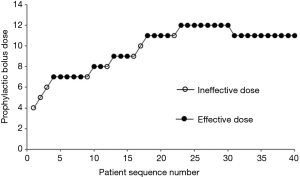
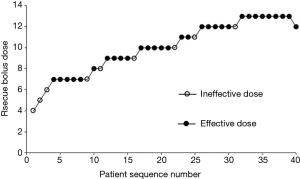
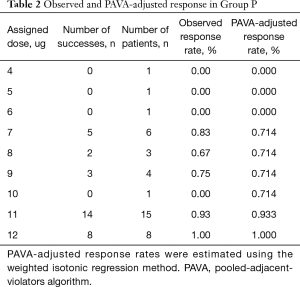
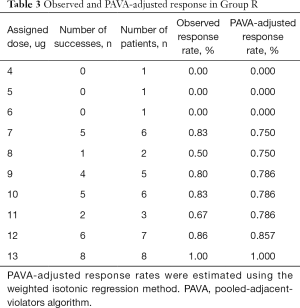
The sequence of effective and ineffective responses at each prophylactic norepinephrine and rescue norepinephrine bolus dose for forty successive patients are shown in Figures 2,3. Tables 2,3 show the observed and PAVA-adjusted response rates for each prophylactic norepinephrine and rescue norepinephrine bolus dose level, respectively. The ED90 value of norepinephrine prophylactic bolus was 10.85 µg (95% CI, 9.20–11.67 µg) and the ED90 value of norepinephrine rescue bolus was 12.3 µg (95% CI, 10.0–12.8 µg) as determined using isotonic regression methods.
Full table
Full table
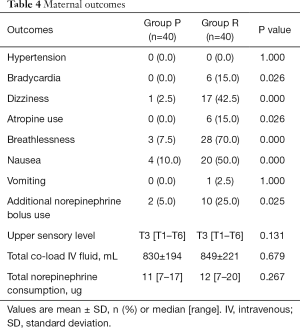
Table 4 shows maternal outcomes. The occurrences of unpleasant symptoms, such as dizziness (2.5% vs. 42.5%, P=0.000), breathlessness (7.5% vs. 70.0%, P=0.000), bradycardia (0.0% vs. 15.0%, P=0.026), and nausea (10.0% vs. 50.0%, P=0.000) related to hypotension were much lower in Group P. The number of patients who needed atropine (0.0% vs. 15.0%, P=0.026) was less in Group P. Fewer patients in Group P needed additional rescue norepinephrine boluses (5.0% vs. 25.0%, P=0.025) to maintain normal blood pressure.
Full table
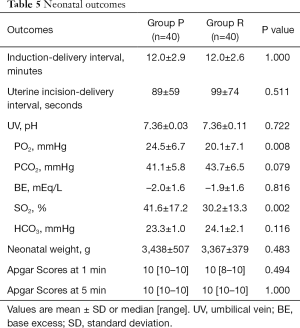
Table 5 shows neonatal outcomes. The results of UV blood gases were not obtained in one patient from Group P and four patients from Group R due to technical problems. The PO2 values of UV (24.5±6.7 vs. 20.1±7.1 mmHg, P=0.008) were higher in Group P. The SO2 values of UV (41.6%±17.2% vs. 30.2%±13.3%, P=0.002) were higher in Group P.
Full table
Discussion
In this sequential dose-finding study, the ED90 value of norepinephrine for both prophylactic and rescue bolus doses to prevent hypotension after CSE during CD were estimated. The results showed that the ED90 value of prophylactic norepinephrine bolus was 10.85 µg (95% CI, 9.20–11.67 µg) and the ED90 value of rescue norepinephrine bolus was 12.3 µg (95% CI, 10.0–12.8 µg) using isotonic regression methods. The occurrences of unpleasant symptoms, such as dizziness, breathlessness, bradycardia, and hypotension related nausea were much lower in Group P. Fewer patients needed atropine or additional norepinephrine boluses in Group P. The PO2 and SO2 values of the UV blood gases were higher in Group P.
The latest study by Onwochei et al. (14) showed the ED90 for prophylactic intermittent bolus doses of norepinephrine, aimed at maintaining patients’ blood pressure at their baseline, was 5.8 µg (95% CI, 5.01–6.59 µg). Intermittent use of this bolus dose was proven to be safe and effective to prevent hypotension after SA. According to Onwochei’s (14) and Ngan Kee’s study (15), a dose of 4 µg was cautiously chosen for the first patient of this sequential study. In Onwochei’s study, the total consumption of norepinephrine ranged from 6 to 78 µg, which was much higher when compared to the current study (11 µg, 7–17 µg). This may partly due to the difference in the study methods. In the Onwochei’s study, the target for the patients’ blood pressure was set as the baseline and the patients were intermittently given a bolus dose of norepinephrine when BP fell below the baseline. To some extent, their treatment of hypotension after SA more approximated a rescue therapy, although it aimed to estimate the ED90 dose of prophylactic norepinephrine. In this study, a real sense of the ED90 of prophylactic norepinephrine was estimated, which caused the increased ED90 value and decreased the total consumption of norepinephrine.
Ngan Kee et al. (15) designed a dose-response study to estimate ED50 and ED90 of norepinephrine to treat the first episode of hypotension after SA. The resulting ED90 dose of norepinephrine was 18 µg, which was higher than that in the current study (12.3 µg). The target BP was also set to 80% of the baseline, the same as in the current study. However, the patients in that study were divided into six different dose groups (4, 5, 6, 8, 10, and 12 µg), and adopted a dose-response method, which was different from the current study. More importantly, the response rate of the 12 µg group was above 95%, which was similar to the result of the current study (12.3 µg). In the dose-response study, the estimated ED90 dose of norepinephrine was 18 µg and this result was based on their special statistic method; however, the dose groups above 18 µg were absent from that study.
It should be noted that the spinal drugs and doses were not same in both studies above (13,14), although the same anesthesia method for the patient of SA was adopted. The CSE was adopted in the current study, which was different from the studies above. The difference of the rate and degree of hypotension in patients was difficult to evaluate due to the prophylactic drug use method, but the median upper anesthetic levels of patients were close (T2 to T4) between the current study, Onwochei’s study, and Ngan Kee’s study. The increasing incidence of hypotension after SA for CD with higher analgesic levels above T4 was proven by several studies (16,17); therefore, we can reasonably infer that the hypotension rates were not much different among three studies.
In the current study, the prophylactic use of a small bolus of norepinephrine was employed in an attempt to prevent the hypotension after SA during CD. It may have more advantages in reduction of unpleasant symptoms related to hypotension and the total consumption of rescue dose of vasopressors, increasing oxygen supplementation for fetuses.
The ED90 dose of norepinephrine in the current study had been proven to be safe by the studies of Onwochei and Ngan Kee. Side effects such as maternal tissue ischemia on hands and forearms, severe maternal hypertension, severe maternal tachycardia, and adverse effect to fetuses and neonates were absent. Only one patient in Group R and two patients in Group P encountered hypertension soon after norepinephrine was given, and all patients’ blood pressures came back to baseline in 3 min. None of them complained about hypertension related side effects such as headaches and nausea. Furthermore, all three patients were more tolerant to anesthesia and had their upper sensory level below T6; therefore, they were excluded from the study. This suggests that identifying the anesthetic effect or upper sensory level before using a bolus of norepinephrine may reduce the occurrence of hypertension.
The current study further proves the effectiveness and safety of prophylactic use of norepinephrine in preventing hypotension after SA during CD. It also provides a new clinical idea of reducing the rate of hypotension, maintaining hemodynamic stability, and reducing the necessary dose of vasopressors by prophylactic use of a small bolus norepinephrine.
Conclusions
The ED90 of a norepinephrine bolus to prevent hypotension and as a rescue bolus for hypotension after CSE during CD was determined to be 10.85 µg (95% CI, 9.20–11.67 µg) and 12.3 µg (95% CI, 10.0–12.8 µg), respectively, using isotonic regression methods. A norepinephrine prophylactic bolus dose of 11 µg or a rescue bolus dose of 12 µg was recommended for clinical practice. Both methods appear to either prevent hypotension or act as a rescue medication for hypotension after CSE, and adverse outcomes were not observed. Furthermore, the prophylactic use of norepinephrine may have more advantages in the reduction of unpleasant symptoms related to hypotension, reducing the likelihood of using rescue drugs, and increasing oxygen supplementation for fetuses than when using a rescue bolus of norepinephrine.
Acknowledgments
The authors would like to acknowledge Professor Hui Zhang (from department of Biostatistics, St. Jude Children’s Research Hospital, Memphis TN, USA) for his introduction Fang Wang to us.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study obtained signed informed consent, and the research plan obtaining approval from the Research Ethics Committee of the International Peace Maternity and Child Health Hospital and registering at http://www.chictr.org.cn (INR-1800016213).
References
- Loubert C. Fluid and vasopressor management for Cesarean delivery under spinal anesthesia: continuing professional development. Can J Anaesth 2012;59:604-19. [Crossref] [PubMed]
- Allen TK, George RB, White WD, et al. A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery. Anesth Analg 2010;111:1221-9. [Crossref] [PubMed]
- Heesen M, Kölhr S, Rossaint R, et al. Prophylactic phenylephrine for caesarean section under spinal anaesthesia: systematic review and meta-analysis. Anaesthesia 2014;69:143-65. [Crossref] [PubMed]
- Bishop DG, Cairns C, Grobbelaar M, et al. Prophylactic phenylephrine infusions to reduce severe spinal anesthesia hypotension during cesarean delivery in a resource-constrained environment. Anesth Analg 2017;125:904-6. [Crossref] [PubMed]
- Nag DS, Samaddar DP, Chatterjee A, et al. Vasopressors in obstetric anesthesia: a current perspective. World J Clin Cases 2015;3:58-64. [Crossref] [PubMed]
- Ngan Kee WD, Khaw KS. Vasopressors in obstetrics: what should we be using? Curr Opin Anaesthesiol 2006;19:238-43. [Crossref] [PubMed]
- Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology 2015;122:736-45. [Crossref] [PubMed]
- Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg 2010;111:1230-7. [Crossref] [PubMed]
- Chen XZ, Chen H, Lou AF, et al. Dose-response study of spinal hyperbaric ropivacaine for cesarean section. J Zhejiang Univ Sci B 2006;7:992-7. [Crossref] [PubMed]
- Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics 2002;58:171-7. [Crossref] [PubMed]
- Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology 2007;107:144-52. [Crossref] [PubMed]
- Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an up-and-down design. Stat Med 2003;22:535-43. [Crossref] [PubMed]
- Diciccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci 1996;11:189-228. [Crossref]
- Onwochei DN, Ngan Kee WD, Fung L, et al. Norepinephrine intermittent intravenous boluses to prevent hypotension during spinal anesthesia for cesarean delivery: a sequential allocation dose-finding study. Anesth Analg 2017;125:212-8. [Crossref] [PubMed]
- Ngan Kee WD. A Random-allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology 2017;127:934-41. [Crossref] [PubMed]
- Kyokong O, Charuluxananan S, Sriprajittichai P, et al. The incidence and risk factors of hypotension and bradycardia associated with spinal anesthesia. J Med Assoc Thai 2006;89 Suppl 3:S58-64. [PubMed]
- Fakherpour A, Ghaem H, Fattahi Z, et al. Maternal and anaesthesia-related risk factors and incidence of spinal anaesthesia-induced hypotension in elective caesarean section: a multinomial logistic regression. Indian J Anaesth 2018;62:36-46. [Crossref] [PubMed]