
The use of nephrometry scoring systems can help urologists predict the risk of conversion to radical nephrectomy in patients scheduled for partial nephrectomy
Conversion to radical nephrectomy (RN) in patients scheduled for partial nephrectomy (PN) is an important outcome that should be strongly considered during the decision-making process and adequately discussed with patients during their preoperative counseling. This unplanned event could be due to severe intraoperative complications or oncologic reasons. Intraoperative suspicion of hilar and/or perirenal fat tissue involvement, peritumoral venous involvement, tumor multifocality and/or iatrogenic rupture of the tumor are the most common conditions threatening the oncological safety of the procedure.
Reported rates of conversion to RN range between 1.4% and 33.5% (1-3). Notably, data extracted from the Premier Healthcare Database in the United States showed that conversion rates declined significantly from 33.5% to 14.5% in the period between 2003 and 2015 (1). Risk of conversion seems to be higher in older patients with high body-mass index and relevant comorbidities (1,2). Moreover, different approaches used by surgeons and annual hospital and surgeon volume seem to play an important role in determining unsuccessful PN (1,3). In particular, laparoscopic PN was associated with a higher risk of conversion to RN in comparison with open PN (OPN) and robot-assisted PN (RAPN) (1). Interestingly, recent RAPN series reported a very low conversion rate, ranging between 1.4% and 5.0% (2,3). Moreover, high-volume surgeons showed significantly lower risk of unsuccessful PN in comparison with their medium- and low-volume counterparts (1,3). Notably, a recent prospective, multicenter study enrolling more than 500 RAPN cases, showed that tumor-related factors, such as clinical stage, tumor location, multifocality, and RENAL nephrometry score, were not associated with an increased conversion risk (2).
Are tumor-related factors truly irrelevant in predicting risk of conversion to RN in patients in whom a PN is planned?
In 2019, Dahlkamp et al. evaluated the ability of RENAL and PADUA nephrometry scoring systems to predict risk of conversion to RN in patients with renal tumors scheduled for OPN or RAPN (4). The authors analyzed a total of 229 patients scheduled for PN in a single institution between January 2013 and May 2017 and reported an overall conversion rate of 13.5%. In detail, conversion rate was 3.7% in RAPN cases and 14.8% in OPN cases. Hilar tumor infiltration (38.7%) and multifocality (22.6%) were the most frequent causes of conversion. Unfortunately, the authors did not clarify the reasons for conversion in the remaining 39.0% of cases.
Looking at preoperative tumor characteristics, 36.2% and 49.3% of the cases were classified as complex cases for PN according to PADUA score ≥10 and RENAL score ≥8, respectively. Conversion rates were 4% and 30% in low- and high-complexity tumors, respectively, defined according to PADUA classification. Similarly, conversion rates were 4% and 23% in low- and high-complexity tumors, respectively, according to RENAL nephrometry system. Interestingly, PADUA score ≥10 [odds ratio 10.98; 95% confidence interval (CI), 4.21–28.60] and RENAL score ≥8 (odds ratio, 7.29; 95% CI, 2.65–20.20) were independent predictors of conversion to RN when adjusted for patient age and comorbidity index.
Notably, Dahlkamp et al. also demonstrated that both nephrometry scoring systems had the same performance in predicting conversion to RN whether calculated by physicians with no specialized radiologic training (i.e., urology residents) or by board-certified radiologists (4). Practical consequences are that urologists can appropriately calculate the nephrometry scores when this information is not included in the computed tomography (CT) scan or magnetic resonance imaging (MRI) radiologic report.
This study deserves some comments. First, the authors must be congratulated because their report represents the first evidence of the role of nephrometry scoring systems in predicting risk of conversion to RN in patients in whom a PN is planned. Moreover, these results further support the accepted role of RENAL nephrometry system (5) and PADUA classification (6) to guide appropriately the decision-making process and the preoperative counseling of patients with renal tumors suitable for PN (7).
Second, data reported by Dahlkamp et al. are conflicting to those previously reported by Arora et al. in a series of 501 patients scheduled for RAPN in 14 different centers involved in the Vattikuti Collective Quality Initiative (2). The two studies reported significantly different conversion rates. While Dahlkamp et al. reported an overall conversion rate of 13.5%, Arora et al. reported a 5.0% rate of unsuccessful PN (2,4). The most relevant issue relates to the different number of complex cases included in the two studies. In the Arora’s study, only 12.5% of cases were classified as high-complexity according to a RENAL score ≥10 (2). Conversely, in the Dahlkamp’s study high-complexity tumors were 36.2% according to PADUA score ≥10 and 49.3% according to RENAL score ≥8 (4). It is reasonable that the different number of events censored and the different proportion of complex cases reported in the two studies could explain the different results reported in terms of impact of nephrometry scoring systems on conversion rate.
Third, nephrometry scoring systems were introduced in 2009 by urologists with the aim to estimate the complexity of PN according to anatomical and topographic characteristics of parenchymal renal tumors (5,6). Most of the parameters included in these systems are not usually described in the final radiologic report, but always evaluated by surgeons in their decision-making process. Nephrometry scoring systems translated a natural mental process of surgeons in a standardized tool able to offer an objective score. Ten years after the introduction of RENAL nephrometry score in USA and PADUA classification in Europe, we do not believe that these systems are widely used by radiologists and are, unfortunately, still omitted from routine radiologic reports. However, urology residents are appropriately trained to assign nephrometry scores in patients candidate for PN. Notably, Dahlkamp et al. reported a moderate concordance (kappa coefficient 0.40) between urology residents and certified radiologists for PADUA classification, and a substantial concordance (kappa coefficient 0.56) for RENAL nephrometry score (4). A higher degree of interdisciplinary concordance could support an easier application of the RENAL nephrometry score in comparison with PADUA classification. Unfortunately, this evaluation was strongly biased by the incorrect cut-off used by the authors to define the high-complexity cases according to the RENAL system. Indeed, Dahlkamp et al. correctly stratified high-complexity cases according to PADUA score ≥10, but they used the cut-off of 8 to stratify cases with the RENAL system (4). According to the original RENAL nephrometry system, high-complexity tumors have a score of 10–12 (5). Therefore, in the Dahlkamp’s study, several medium-complexity tumors were incorrectly considered as high-complexity ones (4). The use of an inappropriate cut-off might facilitate the interdisciplinary concordance when RENAL nephrometry system is assessed in comparison with PADUA classification. Considering the most appropriate definition of high-complexity tumors obtained using the PADUA score ≥10, urology residents correctly scored 71.6% of cases, while underscored 16.2% of cases and upscored the remaining 12.2% of cases. Nevertheless, this disagreement did not impact on the ability of the nephrometry systems to predict the risk of conversion to RN (4).
The interdisciplinary agreement between urologists and radiologists could be further increased using the new simplified version of the PADUA classification, recently proposed by Ficarra et al. (8). Indeed, the Simplified PADUA REnal (SPARE) nephrometry system does not include two variables of the original PADUA score represented by polar location and upper collecting system involvement, which correspond to the most time-consuming steps in partial score assignment (8). Dedicated, prospective studies analyzing the inter- and intra-observer concordance using the SPARE system are eagerly awaited to confirm this hypothesis.
A further tool to improve the correct score assignment of nephrometry systems could be represented by the use of three-dimensional virtual models. Indeed, Porpiglia et al. recently demonstrated that three-dimensional virtual models seem to be more precise than two-dimensional standard imaging to assign PADUA score and to evaluate the surgical complexity of renal tumors (9).
Fourth, although the use of nephrometry scoring systems should be strongly recommended to define tumor complexity in candidates to PN, we must note that recommendations for PN released by international guidelines are still based on clinical tumor size. In the last years, expanding indications of nephron-sparing surgery determined a significant increase in utilization of PN for cT1b and cT2a tumors (10). Not surprisingly larger tumors may be associated with a higher risk of pathological upstaging. Dahlkamp et al. reported a pathologic upstaging in 9% of their patients. In this subgroup with pathological stage >pT1b, conversion rate was 37.5%. This percentage was significantly higher in comparison with that reported for pT1a and pT1b subgroups (7.4% and 26.0%, respectively). However, pathological involvement of fat tissue (pT3a) was observed in only 5.6% of treated cases. In this critical subgroup of patients, in only 40% of cases a conversion to RN was performed. Conversely, in 16% of cases staged as pT1-2 (possibly unnecessary) conversion to RN was performed (4). Interestingly, in a recent multicenter study published by Bertolo et al. including 243 malignant renal tumors scheduled for RAPN for clinically cT2 tumors, conversion rate was only 0.3%. However, final pathologic stage was pT3a and pT3b-4 in 37.0% and 0.8% of cases, respectively (11).
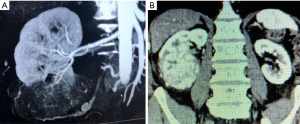
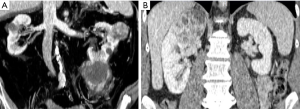
Previous data open an interesting discussion on the ability of surgeons to judge the oncologic safety of PN intraoperatively, and on the safety of their arbitrary decisions to avoid conversion to RN even if signs of non-organ confined disease are present, such as hilar or perirenal fat tissue involvement or presence of peripheral neoplastic venous involvement. This issue appears relevant if we consider that perirenal and/or hilar fat tissue invasion and venous involvement represent unfavorable stages of the disease associated with a significant survival decrease in comparison with pathologically organ-confined disease (12). Indeed, PN is currently not recommended for cT3 tumors, because only a few clinical studies including a limited number of such cases comparing PN and RN are available (13,14). Figure 1 shows two cases directly scheduled for RN according to unfavorable imaging parameters. Figure 2 shows two cases of unsuccessful PN due to multifocal tumors with perirenal fat invasion and to hilar tumor invasion.
Fifth, another interesting data coming from the study published by Dahlkamp et al. is the correlation between conversion rate and histologic tumor characteristics. In particular, conversion rate was 25% in the subgroup of non-clear cell renal cell carcinoma and only 14% in the clear cell subgroup (4). Moreover, conversion rate was 66% in patients with grade 3 tumors versus 22% in grade 2 and 10% in grade 1 tumors. These data suggest that the biology of the disease is another important factor potentially associated with the intraoperative decision of conversion to RN in patients planned for PN.
Very few and conflicting data are available about the ability of nephrometry scoring systems to predict some histologic characteristics of renal tumors (15,16). However, following the same philosophy that promoted the ideation of nephrometry systems, new models could be conceived that combine other imaging features able to predict unfavorable behavior of renal tumors. For example, some urologists empirically consider any of the following features as distinctive of more aggressive tumors: large masses with irregular tumor shape, multiple nodular growth patterns, thin peritumoral capsule, intratumoral necrosis and high amount of intratumoral vessels. Future clinical studies should evaluate the correlation between some of these imaging features and the biology of renal tumors. In this manner, predictors of aggressive behavior may help urologists determine their surgical strategy in favor of immediate RN minimizing the risk of intraoperative conversion in patients not appropriately planned for PN.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Khandwala YS, Jeong IG, Kim JH, et al. The incidence of unsuccessful partial nephrectomy within the United States: a nationwide population-based analysis from 2003 to 2015. Urol Oncol 2017;35:672.e7-672.e13. [Crossref] [PubMed]
- Arora S, Chun B, Ahlawat RK, et al. Conversion of robot-assisted partial nephrectomy to radical nephrectomy: a prospective multi-institutional study. Urology 2018;113:85-90. [Crossref] [PubMed]
- Xia L, Pulido JE, Chelluri RR, et al. Hospital volume and outcomes of robot-assisted partial nephrectomy. BJU Int 2018;121:900-7. [Crossref] [PubMed]
- Dahlkamp L, Haeuser L, Winnekendonk G, et al. Interdisciplinary comparison of PADUA and R.E.N.A.L. scoring systems for prediction of conversion to nephrectomy in patients with renal mass scheduled for nephron sparing surgery. J Urol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. [Crossref] [PubMed]
- Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. [Crossref] [PubMed]
- Klatte T, Ficarra V, Gratzke C, et al. A literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. Eur Urol 2015;68:980-92. [Crossref] [PubMed]
- Ficarra V, Porpiglia F, Crestani A, et al. The Simplified PADUA REnal (SPARE) nephrometry system: a novel classification of parenchymal renal tumours suitable for partial nephrectomy. BJU Int 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Porpiglia F, Amparore D, Checcucci E, et al. Three-dimensional virtual imaging of the renal tumors: a new tool to improve the accuracy of nephrometric scores. BJU Int 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Fero K, Hamilton ZA, Bindayi A, et al. Utilization and quality outcomes of cT1a, cT1b and cT2a partial nephrectomy: analysis of the national cancer database. BJU Int 2018;121:565-74. [Crossref] [PubMed]
- Bertolo R, Autorino R, Simone G, et al. Outcomes of robot-assisted partial nephrectomy for clinical T2 renal tumors: a multicenter analysis (ROSULA collaborative group). Eur Urol 2018;74:226-32. [Crossref] [PubMed]
- Novara G, Ficarra V, Antonelli A, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol 2010;58:588-95. [Crossref] [PubMed]
- Jeldres C, Patard JJ, Capitanio U, et al. Partial versus radical nephrectomy in patients with adverse clinical or pathologic characteristics. Urology 2009;73:1300-5. [Crossref] [PubMed]
- Andrade HS, Zargar H, Akca O, et al. Is robotic partial nephrectomy safe for T3a renal cell carcinoma? Experience of a high-volume center. J Endourol 2017;31:153-7. [Crossref] [PubMed]
- Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol 2011;60:241-8. [Crossref] [PubMed]
- Chapin BF, Wood CG. The RENAL nephrometry nomogram: statistically significant, but is it clinically relevant? Eur Urol 2011;60:249-51; discussion 251-2. [Crossref] [PubMed]


