
From WOEST to AUGUSTUS: a review of safety and efficacy of triple versus dual antithrombotic regimens in patients with atrial fibrillation requiring percutaneous coronary intervention for acute coronary syndrome
Introduction
In patients with atrial fibrillation, the role of anticoagulation has been established to reduce the risk of stroke (1). In patients who undergo percutaneous coronary intervention (PCI), there is clear benefit in utilizing dual antiplatelet therapy (DAPT), especially if a cardiac stent is placed (2). Both of these pharmacologic interventions have been established with multiple randomized controlled trials that provide valuable information regarding agents, dosing, and duration of therapy (1). Due to the vast literature regarding anticoagulation in patients with atrial fibrillation and with DAPT after PCI, there is clear evidence to guide the strategy for antithrombotic therapy when these conditions are independent of each other.
However, in the instance that a patient has an indication for both (i.e., a patient with atrial fibrillation undergoes PCI) providers are faced with a dilemma. The combination of anticoagulation and DAPT, defined as triple therapy, has received great attention in regard to the optimal pharmacotherapy that would provide benefit in preventing thrombotic events (stroke and coronary vessel occlusion) as well as being mindful of the increased risk of bleeding. The purpose of this article is to review prior studies that evaluated and compared the safety and efficacy of dual and triple antithrombotic therapy in patients with atrial fibrillation and a concomitant indication for DAPT.
Methods
A PubMed search was performed using the search terms “antithrombotic”, “atrial fibrillation”, “triple therapy” and “acute coronary syndrome”. This search returned 120 results with articles containing possible combinations of those terms within the title or abstract. Of these 120 articles, 20 were chosen that fit the scope of this review and the list was further narrowed to include articles published after the WOEST trial. An additional search was performed using the name of each trial and “subgroup” as search terms to find articles that may have specifically addressed the acute coronary syndrome (ACS) population. In addition to these trials, several other prior review articles as well as observational studies and consensus recommendations were reviewed.
Vitamin K antagonists
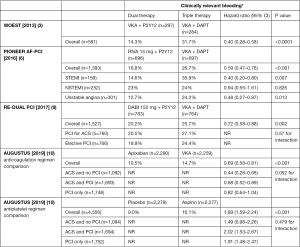
WOEST was the first prospective study to evaluate dual therapy [clopidogrel plus an oral anticoagulant (OAC)] versus triple therapy (aspirin, clopidogrel, and OAC) in patients requiring long-term anticoagulation undergoing PCI (3). The study was a prospective, open-label, randomized controlled trial conducted at 15 centers in Belgium and Netherlands between November 2008 and November 2011. In total, 563 patients were enrolled into one of two groups: 279 patients were assigned to double therapy and 284 patients were assigned to triple therapy. Patients presenting with ACS were a minority of the study population in both the double therapy and triple therapy groups (25% and 30%, respectively). The primary outcome of any bleeding event was significantly decreased in the dual therapy group (19.4%) compared to the triple therapy group (44.4%) at 1-year follow-up. This outcome was consistent across all subgroups, including ACS patients. Dual therapy also reduced TIMI-defined major and minor bleeding by 60% compared to a triple therapy regimen, although this finding was driven primarily by reductions in minor bleeding. Although WOEST was not powered to assess efficacy outcomes, dual therapy significantly reduced the composite endpoint of death, stroke, myocardial infarction, stent thrombosis, and target-vessel revascularization compared to the triple therapy group (11.1% and 17.6%, respectively). Based on the findings of WOEST, treatment of only four patients with a dual therapy regimen is required to prevent a bleeding event, which was consistent in patients presenting with ACS. However, the suggested benefit in the composite efficacy outcome should be interpreted with caution as ACS patients were underrepresented in the study and WOEST was not powered to assess these outcomes.
Observational studies have corroborated the findings of the WOEST trial. A Danish registry of over 12,000 patients with atrial fibrillation undergoing PCI evaluated safety, efficacy, and mortality of a triple therapy regimen compared to OAC with the addition of a single antiplatelet agent (4). Clopidogrel plus OAC showed a lower occurrence of myocardial infarction or coronary death, and a lower incidence of bleeding compared to triple therapy, although neither achieved statistical significance. Of this treatment group, approximately 50% had ACS. OAC plus aspirin significantly reduced bleeds, however this regimen also significantly increased all-cause mortality. These findings are consistent with the WOEST trial, in that just the addition of clopidogrel to vitamin K antagonists is at least equal to triple therapy and perhaps a better option in a patient with a high bleeding risk.
The ISAR-TRIPLE trial was a randomized, open-label trial that enrolled 614 patients from September 2008 through December 2013 (5). Its objective was to determine the optimal duration of triple therapy to mitigate bleeding risk while still providing protection from stent thrombosis after placement of a drug-eluting stent for stable angina or ACS in patients receiving an OAC. Patients were randomized to receive either 6 weeks or 6 months of clopidogrel in addition to daily aspirin and an OAC. The primary endpoint was a composite of death, myocardial infarction, stent thrombosis, stroke or major bleeding at 9 months after randomization. The primary endpoint occurred in 30 of 302 patients (9.8%) in the 6-week group and 27 of 304 patients (8.8%) in the 6-month group. Between the two groups, there was no significant difference in ischemic complications or major bleeding events. Of note, patients with ACS made up only 32% of the study population. The findings of this study suggest that potentially shortening the length of triple therapy may be done without sacrificing efficacy.
Rivaroxaban
As the first large study to evaluate a non-vitamin K oral anticoagulant (NOAC) in this population, PIONEER AF-PCI trial sought to combine the lower bleeding risks of rivaroxaban to standard DAPT therapy to treat patients with atrial fibrillation and ACS/PCI (6). The PIONEER AF-PCI was a multicenter, prospective, randomized controlled trial to evaluate management of patients with nonvalvular atrial fibrillation who recently had PCI. The 2,124 enrolled patients were randomly assigned to 3 treatment groups—P2Y12 plus 15 mg rivaroxaban once daily (Group 1), DAPT plus 2.5 mg rivaroxaban twice daily (Group 2), or DAPT plus warfarin (Group 3). The primary safety endpoint was TIMI-defined major bleeding. Overall bleeding rates at 12 months were reported at 16.8%, 18.0% and 26.7% in groups 1, 2, and 3, respectively. Both groups with rivaroxaban had significantly lower bleeding rates than warfarin group in patients with PCI. Subgroup analyses showed that access site, type of stent, use of closure device, length and number of stents did not modify bleeding risk. Bleeding was significantly lower regardless of the artery affected except for circumflex artery lesions where there was a trend towards lower bleeding (7). The trial reported major adverse cardiovascular events (MACE) in 6.5%, 5.6% and 6.0% in groups 1, 2, and 3, respectively, with no significant difference between the groups. The rate of stent thrombosis was very low and not significantly different among groups. The trial suggested that rivaroxaban-based therapy may be non-inferior in reducing MACE and had lower bleeding risks than standard triple therapy in patients with atrial fibrillation and PCI.
Atrial fibrillation patients undergoing PCI are at increased risk of bleeding and ischemic complications (8). However, atrial fibrillation patients presenting with ACS may not be subject to these increased risks of bleeding and ischemic complications. The PIONEER AF-PCI trial had 52% patients with ACS (6). Clinically significant bleeding rates were significantly lower for the 15 mg rivaroxaban group compared to warfarin group in patients with STEMI (14.6% vs. 35.9%) and unstable angina (12.7% vs. 24.2%). No significant difference was seen in NSTEMI patients (23% vs. 24%) (Figure 1). MACE and time to first stroke had no significant difference in ACS patients in rivaroxaban group or warfarin group (Figure 2).
PIONEER AF-PCI provided evidence of the benefit of low dose rivaroxaban over warfarin in atrial fibrillation patients with PCI. The lower than standard dose of rivaroxaban significantly decreased bleeding complications but had no benefit in MACE compared to warfarin. It is unknown to what effect an FDA approved dose might have resulted for bleeding and ischemic outcomes. Unlike the increased bleeding risks of atrial fibrillation patients undergoing PCI, atrial fibrillation patients with ACS may benefit with this escalated therapy.
Dabigatran
The RE-DUAL PCI study sought to combine a direct thrombin inhibitor, dabigatran, to DAPT in patients with atrial fibrillation who underwent PCI (9). This was a multi-center, prospective randomized controlled trial that was designed to study the safety of using dabigatran with DAPT. The 2,725 patients enrolled were assigned to three treatment groups—dual therapy with dabigatran 110 mg twice daily with either clopidogrel or ticagrelor (Group 1), dual therapy with dabigatran 150 mg twice daily with either clopidogrel or ticagrelor (Group 2), or triple therapy with warfarin along with aspirin plus either clopidogrel or ticagrelor (Group 3).
The primary safety endpoint was the first major or clinically relevant non-major bleeding event defined by the International Society of Thrombosis and Haemostasis (ISTH). The primary outcome occurred in 15.4% in Group 1 and 20.2% in Group 2 compared to 25.7% in Group 3 (P<0.001 and P=0.002, respectively). Bleeding rates were significantly lower in both dabigatran groups compared to triple therapy with warfarin. The secondary outcome of TIMI major bleeding occurred in 1.4% of patients in Group 1 compared to 3.8% in Group 3 (P=0.002). Similarly, TIMI major bleeding occurred in 2.1% of patients in Group 2 compared to 3.9% in Group 3 (P=0.03).
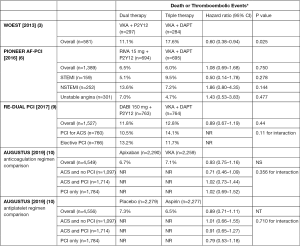
Secondary efficacy outcomes included a combination of thromboembolic events, defined as myocardial infarction, stroke or systemic embolism, death, or unplanned vascularization in addition to definite stent thrombosis among others. While the study was not powered to find differences in efficacy, it is important to note the results. A combination of thromboembolic events, death, or unplanned revascularization occurred in 15.2% in Group 1 compared to 13.4% in Group 3 (P=0.3). While the composite efficacy outcome occurred in 11.8% of the patients in Group 2 compared to 12.8% in Group 3 (P=0.44). A pooled analysis of the rates of thromboembolic events, death, or unplanned revascularization in the dual therapy groups (Group 1 and Group 2) compared to the triple therapy group (Group 3) was performed. Overall, the incidence of this composite outcome occurred in 13.7% of the dual-therapy groups vs. 13.4% in the triple therapy group (P=0.74 for superiority and P=0.005 for non-inferiority). The authors concluded that in patients with atrial fibrillation who required PCI, dual therapy with dabigatran has a lower risk of bleeding compared to triple therapy with DAPT and warfarin, with no significant difference in thromboembolic events.
In the RE-DUAL PCI trial, 50.5% of patients had ACS. The overall findings of the trial were similarly seen in patients with ACS with clinically relevant bleeds occurring in 14.7% of patients in Group 1 compared to 27.8% in in Group 3. Similar results were observed with 20.5% of patients in Group 2 experiencing a clinically relevant vs. 27.1% of patients on triple therapy. Lastly, in a 2019 subgroup analysis of this trial, the benefits of both dabigatran 110 and 150 mg dual therapy compared with warfarin triple therapy in reducing bleeding risks were consistent across subgroups of patients with or without ACS (11).
The RE-DUAL PCI trial provides valuable information that both dabigatran 110 mg twice daily or dabigatran 150 mg twice daily in combination with an antiplatelet, such as clopidogrel or ticagrelor, may be non-inferior in preventing thromboembolic events compared to triple therapy (9). Additionally, dual-therapy with dabigatran resulted in a statistically lower bleeding incidence compared to triple therapy with warfarin. The utilization of dabigatran in this patient population should be based on patient specific bleeding and thrombotic risk.
Apixaban
Most recently, 4,614 patients were randomized in the AUGUSTUS trial to 6 months of either apixaban or vitamin K antagonist, and either placebo or aspirin to assess dual antithrombotic therapy versus triple therapy (10). The anticoagulation assignment was open-label, however, the aspirin or placebo assignment was double-blinded. The design of this trial was novel compared to prior investigations in that atrial fibrillation dosing was utilized for apixaban and comparisons were available for a NOAC and a vitamin K antagonist as part of both a double and triple antithrombotic regimen. The primary outcome of ISTH-defined major bleeding or clinically relevant non-major bleeding occurred in 10.5% of patients in the apixaban group compared to 14.7% in the vitamin K antagonist group [hazard ratio (HR) 0.69; 95% CI, 0.58–0.81; P<0.001]. The percentage of death or hospitalization occurred in 23.5% of patients in the apixaban group and 27.4% in the vitamin K antagonist group (HR 0.83; 95% CI, 0.74–0.93; P=0.002).
Similar results were observed with aspirin versus placebo for the primary safety outcome. ISTH-defined major bleeding or clinically relevant non-major bleeding occurred in 16.1% and 9.0% of patients receiving aspirin and placebo, respectively [hazard ratio (HR) 1.89; 95% CI, 1.59–2.24; P<0.001]. Conversely, there was a lack of benefit for the outcome of death or hospitalization when comparing placebo (24.7%) to aspirin (26.2%). The only ischemic outcome that was associated with greater benefit in either treatment groups, was patients treated with apixaban were 50% less likely to experience a stroke than warfarin-treated patients. The addition or removal of aspirin for this outcome did not influence this outcome.
Of the population included in the AUGUSTUS trial, 1,714 (37.3%) had an ACS event as the reason for inclusion. In subgroup analyses, relative risk reductions of 56%, 32%, and 18% in the primary safety outcome favored apixaban over warfarin in patients with ACS who did not receive PCI, patients with ACS and PCI, and patients with elective PCI, respectively (P=0.052 for interaction). A similar trend was evident for time to death or hospitalization in favor of apixaban in patients with ACS treated medically (HR 0.71; 95% CI, 0.54–0.92).
The authors of the AUGUSTUS trial concluded that the use of apixaban compared to the use of a vitamin K antagonist resulted in a lower rate of bleeding complications as well as a lower composite outcome of death or hospitalization, driven by a reduction in hospitalizations. A higher incidence of bleeding was observed with aspirin use compared to placebo. Again, as with other studies in this review, the proportion of ACS patients was relatively low at just over one-third of the total patient population. Interestingly, apixaban appeared to have better outcomes in patients with ACS treated without PCI in regard to safety and efficacy, however, conclusions drawn from this data is hypothesis generating due to the limited sample.
Discussion
Regarding bleeding events, dual antithrombotic therapy with a P2Y12 inhibitor alone is safer than a triple antithrombotic regimen of aspirin plus a P2Y12 inhibitor and an anticoagulant for atrial fibrillation following PCI (3,4,6,9,10). For patients evaluated in the WOEST trial, the number needed to treat to avoid any bleed or a major bleed was 4 and 40, respectively (3). While a clear safety benefit was evident, there were also no difference in the occurrence of stroke or stent thrombosis. ISAR-TRIPLE demonstrated, at the very least, duration of therapy may be shortened if clinicians are uncomfortable choosing dual antithrombotic therapy from the start when stent thrombosis risk is highest (6). NOACs are recommended over warfarin for the prevention and treatment of thromboembolism because they provide a better safety profile (1,12,13). In the setting of atrial fibrillation with PCI, it is clear after multiple trials that this sentiment holds true, especially with the recent publication of AUGUSTUS (10). The choice of apixaban plus a P2Y12 inhibitor in only 9 patients would prevent a major or clinically-relevant non-major bleeding event, and that same choice in 18 patients would prevent a death or hospitalization, compared to a regimen consisting of vitamin K antagonist plus DAPT. Yet many clinicians are hesitant to make this choice for several reasons.
In the landmark trials evaluating NOAC therapy as a substitute to warfarin, there has been much debate about interpretation of results. PIONEER AF-PCI, as expected, described reduced bleeding in both rivaroxaban arms with low dose (15 mg) and very low dose (2.5 mg) regimens of which neither are FDA approved doses for atrial fibrillation (6). RE-DUAL PCI also displayed a better safety profile of dual therapy regimens consisting of dabigatran 110 or 150 mg twice daily (9). Similarly, concerning the limitations of PIONEER AF-PCI, the dabigatran 110 mg dose is not FDA approved for stroke prevention in atrial fibrillation and as expected, the lowest rates of bleeding were evident with this regimen. On the other hand, dabigatran 110 mg was studied in the RE-LY trial, which was non-inferior to warfarin for efficacy and superior for bleeding outcomes, which may allow clinicians a higher level of comfort in clinical practice knowing there is evidence established (14). The authors of these studies acknowledge that these trials are not powered to evaluate ischemic or thromboembolic outcomes, which further elevates concerns regarding off-label dosing strategies. The OBRBIT-AF II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation phase II) registry suggests patients who are not receiving appropriate dosing based on FDA labeling are at an increased risk of cardiovascular hospitalization (15). AUGUSTUS answers these concerns by utilizing FDA approved dosing and proving that apixaban-based regimens reduces hospitalization compared to vitamin K antagonist-based regimens (10). The overall safety profile of apixaban and available evidence should quiet these concerns and potentially result in a stronger evidence recommendation when guidelines are updated.
The other main concern is the nature of these trials to combine elective and urgent PCI for ACS. Not only have these studies focused on reduction of bleeding outcomes and thus underpowered for ischemic outcomes, the lack of focus on the ACS population further clouds judgment on how to treat the highest-risk patients. The prevalence of ACS in the various trials range from 28% to 61% (16). It is likely that triple therapy will continue to be prescribed, even if for a shortened duration, due to concern of risk of ischemic outcomes. In a registry of approximately 5,000 patients with MI and atrial fibrillation, 1 in 4 patients were prescribed triple therapy, which doubles the risk of intracranial hemorrhage yet did not improve MACE (adjusted HR 0.99; 95% CI, 0.86–1.16) (17). The evidence suggests that dual antithrombotic therapy is likely acceptable in an ACS population, however, additional randomized trials that specifically address this population or future meta-analysis are needed. More patients will be available for this method when the results for edoxaban in the ENTRUST- AF PCI study is published in the near future (18).
Conclusions
There exist many therapeutic options to prevent thromboembolic events in patients with atrial fibrillation who undergo PCI with stenting. For patients with stable coronary artery disease and receive elective PCI, the choice is clear that a dual antithrombotic regimen of a P2Y12 inhibitor in combination with a NOAC carries the least risk in terms of safety (16). In patients with ACS, the safety benefit remains clear, however questions persist whether there is a potential to sacrifice efficacy. After a thorough review of these studies, there is no signal to suggest that this is in fact true. The AUGUSTUS trial, presents the strongest research design and data to support these regimens. AUGUSTUS is the only trial to show a significant benefit in stroke reduction in such a short time (11). Also, AUGUSTUS was the first to include ACS patients who were medically managed, which is intriguing as these patients are often older and may be at higher bleeding risk. Ultimately, further studies are necessary in larger populations with a majority of ACS patients. Until then, in similar fashion to current recommendations with dabigratran, rivaroxaban, and warfarin, it is reasonable to use full-dose apixaban in addition to a P2Y12 inhibitor in patients with atrial fibrillation who have an ACS event, especially in patients who have a propensity for bleeding (1).
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Cave discloses that he serves on the speaker’s bureau for Portola Pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104-32. [Crossref] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44-122. [Crossref] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981-9. [Crossref] [PubMed]
- Fiedler KA, Maeng M, Mehilli J, et al. Duration of Triple Therapy in Patients Requiring Oral Anticoagulation After Drug-Eluting Stent Implantation: The ISAR-TRIPLE Trial. J Am Coll Cardiol 2015;65:1619-29. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Kerneis M, Gibson CM, Chi G, et al. Effect of procedure and coronary lesion characteristics on clinical outcomes among atrial fibrillation patients undergoing percutaneous coronary intervention: insights from the PIONEER AF-PCI trial. JACC Cardiovasc Interv 2018;11:626-34. [Crossref] [PubMed]
- Mehran R, Kalkman DN, Angiolillo DJ. Atrial fibrillation, with ACS and PCI: walking a tightrope. Eur Heart J 2019;40:1563-6. [Crossref] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513-24. [Crossref] [PubMed]
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509-24. [Crossref] [PubMed]
- Oldgren J, Steg PG, Hohnloser SH, et al. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur Heart J 2019;40:1553-62. [Crossref] [PubMed]
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018;154:1121-201. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin k antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 2016;68:2597-604. [Crossref] [PubMed]
- Lopes RD, Hong H, Harskamp RE, et al. Safety and Efficacy of Antithrombotic Strategies in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Network Meta-analysis of Randomized Controlled Trials. JAMA Cardiol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Hess CN, Peterson ED, Peng SA, et al. Use and Outcomes of Triple Therapy Among Older Patients With Acute Myocardial Infarction and Atrial Fibrillation. J Am Coll Cardiol 2015;66:616-27. [Crossref] [PubMed]
- Vranckx P, Lewalter T, Valgimigli M, et al. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: Rationale and design of the ENTRUST-AF PCI trial. Am Heart J 2018;196:105-12. [Crossref] [PubMed]


