
Effect of sevoflurane and propofol on acute kidney injury in pediatric living donor liver transplantation
Introduction
Pediatric liver transplantations (LT) are becoming increasingly common for treating end stage liver disease in children. Acute kidney injury (AKI) is one of the most common postoperative complications that occurs after LT, accounting for an incidence of up to 40% to 70% (1,2). This is associated with adverse outcomes such as prolonged hospital stay, high morbidity and mortality rate and increased risk of progression to chronic kidney disease (3,4). However, in contrast to adult LT, data regarding the incidence of the factors associated with AKI outcomes in pediatric LT recipients are scarce.
The pathogenesis of LT-associated AKI remains complex, and involves hemodynamic changes that result from suprarenal inferior vena cava occlusion, ischemia-reperfusion injury, and pharmacologic agents influencing renal function. This may in turn cause oxidative stress and inflammation in the kidney (5,6). Several studies have revealed that inflammatory reactions and oxidative stress plays an important role in the initiation and extension phases of AKI (7,8). Furthermore, neutrophil gelatinase-associated lipocalin (NGAL), an early predictive biomarker of AKI, has been successfully used in both cardiac as well as transplantation studies (9).
Proper use of anesthetics during perioperative period benefited those patients undergoing organ transplantation, as they contribute to organ protection through different signaling pathways. Propofol and sevoflurane are widely used general anesthetics, which modulate the inflammatory and oxidative stress responses to surgical stimulations in both clinical and experimental studies (10,11). It is still unclear as to which anesthetic more effectively prevents postoperative AKI after pediatric LT. Hence, this study aimed to evaluate the renal effects of propofol and sevoflurane in pediatric LT patients through changes in systemic hemodynamics, the levels of inflammatory and oxidative biomarkers and NGAL levels.
Methods
Participants
This study has been approved by the ethics committee of Tianjin First Center Hospital in China (Approval Number: 2016N0039KY) and written informed consent was obtained from eligible guardians. All processes conducted were according to the approved protocol.
Eligible children (aged 5 months–2 years; and American Society of Anesthesiologist physical status III to IV) between October 2016 and October 2017 underwent elective pediatric living related donor LT. Exclusion criteria were as follows: pediatric patients with (I) known or suspected allergy to propofol, soy, or egg; (II) congenital heart disease; (III) impairment of renal and/or pulmonary function before LT; and (IV) complexity due to other site operations. All living donors were from their family members (either from the father or mother side). Every case of transplantation has passed the ethical review and approval from the Tianjin First Center Hospital.
Anesthesia and design
Patients enrolled in this study were randomly assigned to pediatric related LT with either intravenous anesthetic propofol or volatile anesthetic sevoflurane. All patients were randomly assigned to the groups by a computer-generated random number system and individually sealed in envelopes. Patients were blinded to the group assignment. Primary and secondary outcomes were analyzed and documented by another anesthesiologist responsible for data collection, but not directly involved in the treatment of patients and blinded to randomization. No patient received any premedication. Anesthesia was induced using scopolamine (0.01 mg/kg), midazolam (0.15 mg/kg), etomidate (0.15 mg/kg), fentanyl (2–5 µg/kg) and vecuronium (0.2 mg/kg) to maintain analgesia, muscle relaxation and sedation. In the propofol group, anesthesia was maintained with propofol (9–15 mg·kg−1·h−1), atracurium besylate (1–2 µg·kg−1·min−1) and fentanyl (1–2 µg/kg). In the sevoflurane group, propofol infusion was replaced by sevoflurane (0.6–1.5 MAC) till the end of the operation. A bispectral index score was maintained between 40 and 60 during anesthesia. Invasive hemodynamic monitoring including pressure measurement by arteria radialis puncture and right internal jugular vein puncture were performed. In the clinical process, we maintain the target blood pressure by continuous infusion of dopamine, combined with epinephrine, nitroglycerin and norepinephrine (maintained the systolic blood pressure at 70–90 mmHg and diastolic blood pressure at 2/3 systolic blood pressure based on the age of child). Also the albumin and the blood product were infused to maintain blood volume and hemoglobin at 8 g/L or more. The electrolyte and acid-base balance were kept within the normal range during surgery. All patients were routinely admitted to the transplantation intensive care unit (ICU) and were intubated. No immediate extubation was performed at the end of the surgery.
Surgery
The operative procedure was performed by using both caval replacement and Piggyback technique. Reperfusion of the liver started with the opening of the portal vein, followed by opening of the artery. After arterial reperfusion, the bile duct was connected either to the recipients’ bile duct (choledocho-choledochostomy) or to a small-bowel loop (hepatico-jejunostomy). A back table biopsy of the donor liver was performed before implantation.
Blood assays
Venous blood (3 mL) was collected from the right internal jugular catheter and placed into the vacuum tubes containing sodium heparin. The blood samples were collected at 6 time points: just before induction of general anesthesia (baseline, T1), 5 min after anhepatic phase (T2), 10 min after reperfusion of hepato-reperfusion (T3), 2 h after reperfusion of the new liver (T4), 24 hours after surgery (T5) and 3 d after surgery (T6). Samples were placed in dry tubes and then were centrifugated. The serum was removed and stored at −80 °C till the analysis. The serum creatinine (SCr), serum neutrophil gelatinase–associated lipocalin (NGAL), interleukin-18 (IL-18), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), hydrogen peroxide (H2O2), malondialdehyde (MDA) and superoxide dismutase (SOD) were analyzed at the above mentioned time points. We used Wuhan Huamei Biological Technology Company (Wuhan, China) for constructing the reaction standard curves. The protein levels were calculated while comparing the optical density values of the samples with the standard curve.
Data collection
The following patients and preoperative variables were included: patient characteristics such as age, gender, height, weight, pediatric end-stage liver disease (PELD), preoperative laboratory test results, and so on. Intraoperative data including graft cold ischemia time, operative time, urine output, bleeding volume, blood products and fluid transfusions were collected. Postoperative data including the laboratory test results, the ICU stay time, ICU-mortality, hospitalization time, and in-hospital mortality were collected.
Renal function assessment
The primary outcome was AKI, and was defined as any stage of AKI according to the Kidney Disease: Improving Global Outcomes guidelines (12); increase in SCr by ≥0.3 mg/dL (≥26.5 µmol/L) within 48 hours; orincrease in SCr of ≥1.5 times than that of the baseline, which is known or presumed to have occurred within or prior to 3 days; or urine volume of <0.5 mL/kg/hour for 6 hours. The AKI diagnoses were based on SCr concentration measured daily for 3 consecutive days after surgery. The most recent SCr level measured before the surgery was used as the baseline value.
Statistical analysis
Statistical analyses were performed by using SPSS 23.0 software package for Windows (SPSS, IBM., Chicago, IL, USA). Kolmogorov-Smirnov was used to analyze the distribution of data. Results are presented as means (standard deviations) or medians (interquartile ranges) or number of patients. Patient characteristics and perioperative clinical data in the 2 study groups were compared by using independent t-test or Fisher’s exact test, as appropriate. Group comparisons of hemodynamic variables and renal biomarkers at each time point were analyzed by using an independent t-test. Changes in group hemodynamic variables and renal biomarkers over time were analyzed by using ANOVA followed by appropriate post hoc test. Comparison of count data was used with c2 test or by utilizing Fisher’s exact method. The results were evaluated within 95% reliability index, with P<0.05 being significant.
Results
With the incidence of AKI in children after LT of 46% (13), and assumption of 25% reduction at an α-error of 0.05 and a ß-error of 80%, at least 58 patients should be included per group, with an expected drop out rate of 10%. From October 2016 to October 2017, 151 pediatric patients underwent living-donor LT. Of these, 31 were excluded, due to 2 repeated operations in 2 patients, hepatic coma in 4 patients, combined operations on parts other than large or small bowel in 3 patients, anesthetized with anesthetics other than sevoflurane or propofol (3 with desflurane, and 5 with intravenous-inhalation combined anesthesia) in 8 patients, and no availability of preoperative or postoperative Scr values in 12 patients. Thus, a total of 120 patients were included in the final analysis (Figure 1).
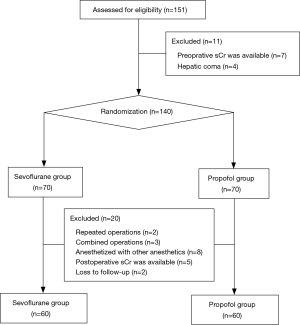
Patient characteristics and intraoperative data
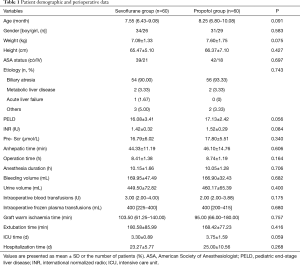
A total of 120 patients were included in the final analysis. The median follow-up for the overall patient population was 0.8 (IQR, 0.3–2) years. Patient characteristics and intraoperative data according to the anesthetic type are summarized in Table 1. Patient characteristics, total operative times, anhepatic time, and urine output data were similar in both the groups. Similarly, no significant intergroup differences were observed between epinephrine, and dopamine requirements. No diuretics were used during and after the surgery.
Full table
The primary outcome-AKI incidence rate
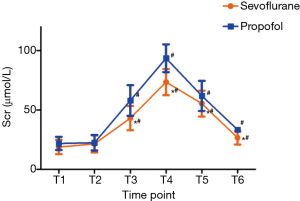
The incidence of AKI during the perioperative period was significantly lower in the sevoflurane group than that of the propofol group [28% (17/60) vs. 47% (28/60), P<0.05]. Changes in Scr were illustrated in Table 2 and Figure 2. No intergroup differences in Scr values were observed at T1 and T2.Scr was increased significantly at T3–6 versus T1 in both the groups (all P values <0.01) and peaked at T4. However, Scr was significantly lowered in sevoflurane group than in the propofol group at T3–6. No patient received prolonged mechanical ventilation (over 48 hours) or stayed in the ICU for >1 week. Furthermore, no patient required renal replacement therapy or diuretics during and after the surgery.

Full table
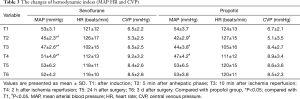
Perioperative hemodynamic changes
Hemodynamic variables were presented in Table 3. No intergroup differences in HR or CVP were observed at any time point. Compared with T1, the value of MAP was significantly decreased at T2–4 and remained the lowest at T2 (P<0.05). Notably, MAP was higher in the sevoflurane group than in the propofol group at T2–4 (P<0.05). HR and CVP over time showed no significant differences between the 2 groups.
Full table
The differences of inflammatory factors and oxidative biomarkers
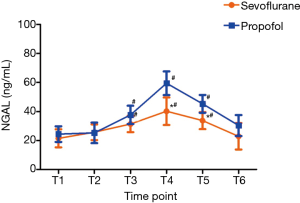
The inflammatory factors of IL-10, IL-18, TNF-α and NGAL were shown in Table 4, and the trend of NGAL was shown in Figure 3. Mean values of IL-10, IL-18, TNF-α and NGAL reached a maximum at T4 and thereafter showed a gradual reduction at T5 and T6 in both the groups. The levels of IL-18, TNF-α and NGAL were significantly lower in the sevoflurane group than in the propofol group at T3–5 (P<0.05), and the levels of IL-10 showed no statistical differences between the two groups.
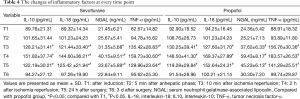
Full table
The indexes of oxidative biomarkers including H2O2, MDA and SOD in the serum were increased at T2 versus T1 and reached a maximum value at T4. The results showed that the levels of these oxidative biomarkers were increased significantly at T3–5 when compared to T1. No intergroup differences of oxidative biomarkers were observed atany time point (Table 5). Excessive oxidative stress might be one of the reasons caused due to AKI.
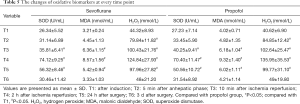
Full table
Discussion
Despite several advances in LT procedures, the incidence of postoperative AKI has been unchanged from over the past few decades. Several studies have indicated that postoperative AKI is a significant risk factor for the poor prognosis after LT (2,6,14,15). Furthermore, adults and children differ in terms of etiology of the kidney, and the epidemiological features of AKI after pediatric LT might differ from those after adult LT. Therefore, it is of great clinical significance to identify potentially modifiable perioperative factors to prevent or ameliorate AKI in pediatric liver patients.
The existing conventional biomarkers of kidney function included SCr, blood urea nitrogen (BUN) and urine output, which are currently utilized for diagnosing and staging of AKI (16). In this study, we found a lower incidence of AKI in the sevoflurane group according to KDIGO when criteria compared with propofol group (28% and 47%, respectively). Of note, our study found that at 5 min of neohepatic phase, the levels of SCr began to increase obviously, reaching a maximum value (1.5-fold of baseline) at 2 h of neohepatic phase. This indicated that the renal function was impaired after reperfusion during the surgical procedure. There are two main reasons for the impairment of renal function: (I) the blood flow in renal venous return is limited because of the blockage of inferior vena cava during anhepatic phase, which cause kidney congestion; (II) the ischemia reperfusion syndrome (anaerobic metabolism in following limbs, intestinal flora shift and the entry of bacteriocin into the blood) cause the secondary to the kidney. Interestingly, the Scr levels of pediatric recipients in sevoflurane group were notably decreased when compared with those of the propofol group, although the remaining showed a 50% increase dramatically. This suggested that the renal protection effect of sevoflurane may be superior over propofol in pediatric LT, implying that sevoflurane anesthesia was associated with earlier renal recovery.
Perioperative anesthesia-related variables including anemia and ischemia reperfusion, hemodynamic fluctuation, and surgical stress response (2,17) were associated with AKI in transplantation patients. Kidney is an exceptionally sensitive organ to changes in vascular compliance and perfusion, making AKI a frequent complication of progressive liver disease. The blood pressure decreased after blocking the inferior vena cava in clinical. The blood flow to the heart reduced and heart ejection decreased because of the blocking of inferior vena cava during the anhepatic phase. Vasoactive drugs such as dopamine and norepinephrine and blood products were used to increase blood pressure so that the blood pressure is reduced by no more than 2 minutes. Moreover, we have made statistics on the application of vasoactive drugs and blood products, and found no difference in the total amount of application between the two groups. In this study, the results revealed that the hemodynamics of patients in the sevoflurane group when compared with those of propofol group at the time of liver reperfusion were steady. In particular, the MABP level in sevoflurane anesthesia group was changed to maintain a ±9.5% of the basic line from beginning till end, and the differences showed no significance. On the contrary, propofol infusion purely resulted in obvious variations of MABP at a level of ±12.7%. This may partly explain as to why sevoflurane anesthesia showed better renal function than propofol in pediatric LT patients.
Recent studies have reported that markers of oxidative stress and inflammation are associated with AKI (18,19). Due to major surgical trauma and complex pathological physiology in LT, oxidative stress and inflammation were intimately related to perioperative anesthesia management and long term prognosis. The pro-inflammatory cytokines, TNF-α and IL-18, were identified as mediators of ischemic injury in the kidney and have been shown to be reliable markers of and contributors to AKI (20). IL-10 is a potent inhibitor of monocyte/macrophage function, suppressing the production of many pro-inflammatory cytokines (21). NGAL is a gene that has been shown to be dramatically upregulated and expressed in the renal tissues during ischemia. It is considered as a potentially useful marker for the detection of early renal tubular injury in renal transplantation as well as LT patients (22). The results in our study suggested that after liver reperfusion, the inflammatory mediator including TNF-α, IL-18 and IL-10 and levels of NAGL were increased dramatically in the serum. Unexpectedly, the levels of TNF-α, IL-18 and NAGL in sevoflurane group were significantly lowered than those under propofol anesthesia. However IL-10 levels showed no difference between both the groups. Apparently, sevoflurane when compared with propofol might be in favor of providing renal protection in pediatric LT patients.
Oxidative stress has also been reported to be a contributing factor to renal injury, especially during the early stages of ischemia-reperfusion (23). Some oxidative biomarkers, such as SOD, MDA, and H2O2 were used as valuable indicators of oxidative stress (24,25). SOD is a major antioxidant enzyme that contributes to the destruction of free superoxide radicals and other reactive oxygen species, blocking free radical-induced damage in the body (26). MDA is a marker of free radical species-related injury, and H2O2 is the main reactive oxygen species produced. Of note, these oxidative stress mediators were dramatically increased when compared with the basic data in the neohepatic phase, reflecting the degree of oxidative damage and acute renal injury (27). However, the levels of increased mediators showed no differences between both the groups. Therefore, the two anesthetic drugs had identical anti-oxidative properties in pediatric LT patients.
However, our study has several limitations that need to be acknowledged. This study is a single-center, randomized controlled study with a small sample size. Further large, multicenter prospective trials are needed to establish such association. Secondly, the serum examination might be a sensitive and poor specificity test, indicating early and minor changes in kidney function and is affected by muscle wasting and ascites (28,29). Recent studies have put forwarded that urine biomarkers including inflammatory and oxidative factors might also be considered as early indicators of AKI following LT (30).
In conclusion, to the best of our knowledge, this is the first study to compare the incidence of postoperative AKI under different anesthetic agents after pediatric LT. Above all, sevoflurane anesthesia may be considered as a way to attenuate renal injury by stabilizing the hemodynamic changes, regulating oxidative stress and inflammatory action and so on. Especially, NAGL, as a useful marker, maybe useful for the detection of early renal tubular injury in renal transplantation as well as LT patients.
Acknowledgments
Funding: This work was supported by research grants: Natural Science Foundation of Tianjin (17JCYBJC28000), Science and Technology Foundation of Tianjin Health Bureau (13KG105, 16KG101), Integrated Western and Chinese Science Foundation of Tianjin Health Bureau (2017056), Project of Young and Middle-aged Scientific Fund of Tianjin Medical Association Anesthesiology Branch in 2017 (TJMZJJ-2017-01), Tianjin Municipal Bureau Science and Technology Fund (2015KZ027).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study has been approved by the ethics committee of Tianjin First Center Hospital in China (Approval Number: 2016N0039KY) and written informed consent was obtained from eligible guardians. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Lewandowska L, Matuszkiewicz-Rowinska J. Acute kidney injury after procedures of orthotopic liver transplantation. Ann Transplant 2011;16:103-8. [Crossref] [PubMed]
- Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015;114:919-26. [Crossref] [PubMed]
- Aydin SI, Seiden HS, Blaufox AD, et al. Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg 2012;94:1589-95. [Crossref] [PubMed]
- Taylor ML, Carmona F, Thiagarajan RR, et al. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J Thorac Cardiovasc Surg 2013;146:146-52. [Crossref] [PubMed]
- Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol 2011;174:119-28. [Crossref] [PubMed]
- Leithead JA, Armstrong MJ, Corbett C, et al. Hepatic ischemia reperfusion injury is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int 2013;26:1116-25. [Crossref] [PubMed]
- 7 Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009.137072.
- Fassett RG, D’Intini V, Healy H, et al. Assessment of arterial stiffness, oxidative stress and inflammation in acute kidney injury. BMC Nephrol 2009;18;10:15.
- Kiani AN, Wu T, Fang H, et al. Urinary vascular cell adhesion molecule, but not neutrophil gelatinase-associated lipocalin, is associated with lupus nephritis. J Rheumatol 2012;39:1231-7. [Crossref] [PubMed]
- Yoo YC, Shim JK, Song Y, et al. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int 2014;86:414-22. [Crossref] [PubMed]
- Kong HY, Zhu SM, Wang LQ, et al. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol 2010;32:347-55. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl 2012;2:1-138.
- Hamada M, Matsukawa S. Acute kidney injury after pediatirc liver transplantation: incidence, risk factors, and association with outcome. J Anesth 2017;31:758-63. [Crossref] [PubMed]
- Nadeem A, Salahuddin N, El Hazmi A, et al. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Crit Care 2014;18:625. [Crossref] [PubMed]
- Leithead JA, Rajoriya N, Gunson BK, et al. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol 2014;60:1180-6. [Crossref] [PubMed]
- McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 2013;182:13-29. [Crossref] [PubMed]
- Hamada M, Matsukawa S, Shimizu S, et al. Acute kidney injury after pediatric liver transplantation: incidence, risk factors, and association with outcome. J Anesth 2017;31:758-63. [Crossref] [PubMed]
- Sahu BD, Kuncha M, Sindhura GJ, et al. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 2013;20:453-60. [Crossref] [PubMed]
- Lee PY, Chien Y, Chiou GY, et al. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant 2012;21:2569-85. [Crossref] [PubMed]
- Dalboni MA, Quinto BM, Grabulosa CC, et al. Tumour necrosis factor-a plus interleukin-10 low producer phenotype predicts acute kidney injury and death in intensive care unit patients. Clin Exp Immunol 2013;173:242-9. [Crossref] [PubMed]
- Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 2009;13:R104. [Crossref] [PubMed]
- Niemann CU, Walia A, Waldman J, et al. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl 2009;15:1852-60. [Crossref] [PubMed]
- Qiao X, Li RS, Li H, et al. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am J Physiol Renal Physiol 2013;304:F112-9. [Crossref] [PubMed]
- Kim JY, Lee JW, Youn YJ, et al. Urinary levels of 8-iso-prostaglandin f2a and 8-hydroxydeoxyguanine as markers of oxidative stress in patients with coronary artery disease. Korean Circ J 2012;42:614-7. [Crossref] [PubMed]
- Radovanovic S, Savic-Radojevic A, Pljesa-Ercegovac M, et al. Markers of oxidative damage and antioxidant enzyme activities as predictors of morbidity and mortality in patients with chronic heart failure. J Card Fail 2012;18:493-501. [Crossref] [PubMed]
- Vacek TP, Gillespie W, Tyagi N, et al. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids 2010;39:1161-9. [Crossref] [PubMed]
- Tesch GH. Review: serum and urine biomarkers of kidney disease:a pathophysiological perspective. Nephrology (Carlton) 2010;15:609-16. [Crossref] [PubMed]
- Li Y, Zhu M, Xia Q, et al. Urinary neutrophil gelatinase associated lipocalin and L-type fatty acid binding protein as diagnostic markers of early acute kidney injury after liver transplantation. Biomarkers 2012;17:336-42. [Crossref] [PubMed]
- Cruz DN, Bagshaw SM, Maisel A, et al. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol 2013;182:45-64. [Crossref] [PubMed]
- Liu D, Huang P, Li X, et al. Using inflammatory and oxidative biomarkers in urine to predict early acute kidney injury in patients undergoing liver transplantation. Biomarkers 2014;19:424-9. [Crossref] [PubMed]


