
Diagnostic tools in late onset Pompe disease (LOPD)
Introduction
Pompe disease (glycogen storage disease type II, OMIM#232300) is an inherited metabolic disorder due to deficiency of acid alpha-glucosidase (GAA) that acts within lysosomes and is responsible of glycogen breakdown to glucose (1-3).
Glycogen accumulates in the lysosome as well as in the cytoplasm leading to tissue damage both directly and by affecting different downstream metabolic pathways including the autophagic process. Although cardiac and skeletal muscles are the main tissues involved, GAA deficiency is ubiquitous, and nowadays, Pompe disease is considered a multisystem disorder (4-6).
Based on the age at onset, Pompe disease can manifest as a severe infantile form (IOPD) presenting with cardiac hypertrophy, respiratory dysfunction and floppiness, and as a late onset form (LOPD) that is more benign and more heterogeneous with respiratory and skeletal muscles involvement (2).
In LOPD, the first clinical manifestation can be either proximal muscle weakness or other complains such as exercise intolerance, muscle pain or even isolated hyperCKemia. The clinical presentations are similar to those in other hereditary or acquired muscle disorders such as, for example, limb-girdle muscular dystrophies (LGMD), other muscle glycogenosis, and inflammatory myopathies (7,8).
For over a decade, enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (rhGAA) has been the only specific therapy for the disease. Several studies demonstrated the efficacy of ERT mainly in IOPD, but it became evident that early initiation of ERT is essential to avoid irreversible muscle damage (9,10).
A recent European Pompe Consortium (EPOC) listed some recommendations on diagnosis and management of Pompe disease and proposed the detection of GAA activity in dried blood spots (DBS) as a rapid and appropriate first line diagnostic test (11). Different studies worldwide have shown that this method is very useful for fast screening of LOPD high-risk populations and should play a central role in the diagnostic algorithm (12-15). To confirm the diagnosis, it is also recommended to identify GAA deficiency in other tissue such as leucocytes, fibroblasts, or skeletal muscle and/or perform Sanger GAA sequencing for genetic diagnosis (13).
Detection of lymphocytes with vacuoles filled with glycogen in the blood smear (BSE; blood smear examination) has been suggested as a useful tool for Pompe diagnosis (16-19).
The use of muscle biopsy as a diagnostic method for Pompe disease has been controversial but, in LOPD, this procedure remains an important tool in the diagnostic process. The biopsy can show some specific myopathic changes with vacuolated myofibers and glycogen accumulation inside and outside the vacuoles; however, in some cases only minimal abnormalities can be found (20).
The aim of the present review is to discuss the main tools currently used in the diagnostic workup of patients suspected of having Pompe disease.
Clinical aspects
The clinical picture of LOPD is rather non-specific. It is usually characterized by muscle weakness, often more prominent in lower limbs. A restrictive respiratory insufficiency, mainly due to diaphragmatic weakness can sometime be the first presentation, but it is invariably associated with skeletal muscle myopathy (21). The increased awareness of the disease has allowed to improve the diagnosis, thus reducing the diagnostic delay and identifying the patients with mild signs and symptoms such as myalgia, fatigue or isolated hyperCKemia. A multisystem involvement has been recently reported (22,23).
Electrophysiological findings
In LOPD, where skeletal muscle involvement is a prominent feature, it is useful to perform electrophysiological studies to consider differential diagnosis with other neuromuscular disorders. So far, few studies have systematically described electromyography (EMG) features and their distribution in LOPD patients. EMG study revealed a myopathic pattern with myotonic discharges (MD) or fibrillation potentials; MD seem more common in paraspinal muscles and tensor fasciae latae, suggesting that these muscles should be included during EMG of suspected cases (24,25).
Laboratory investigations
Routine blood test
Laboratory examination shows increased levels of muscle enzyme such as CK, LDH, AST, and ALT. HyperCKemia is often no more than five-fold from reference ranges and is usually higher in juvenile patients compared to those with a longer disease duration. Elevated CK can be found in some presymptomatic patients. On the other hand, some symptomatic LOPD patients can have normal CK values.
GAA activity assay
The gold standard in the diagnosis of Pompe disease is the detection of GAA deficiency. GAA is a ubiquitous lysosomal enzyme and its activity can be assayed in tissues; the use of blood-based assays has been increasing over time because they are less expensive and less invasive but still reliable and accurate (26) (Table 1).
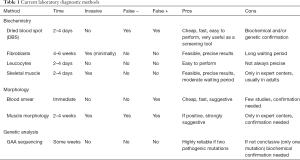
Full table
Dried blood spots (DBS)
GAA activity assay in dry blood spotted (DBS) on filter paper is a very cheap, fast and efficient test to use as a first choice for screening. DBSs are stable during transport and can be easily shipped to specialized laboratories for diagnosis (26). Currently two different methods are used to analyze DBS samples: fluorometry or tandem mass spectrometry. These techniques, applied in different laboratories, are both suitable for Pompe diagnosis, even when applied in newborn screening programs (NBS).
Several studies in target populations revealed the key role of DBSs in the diagnostic work-up of Pompe disease (12-15). In a large cohort of patients with unclassified LGMW and/or hyperCKemia, the prevalence of adult Pompe disease is about 2.0% with slight differences among different countries. For instance, the frequency of Pompe disease among African-Americans, Dutch, and Taiwanese appears to be higher compared to other populations (15).
A false positive DBS test can occur because of incorrect blood spotting and sampling and environmental circumstances such as high temperature during transport. Although the measurement of neutral maltase activity is performed in all DBS assays (an internal control), there is still a possibility of a false-positive result; therefore, it is recommended that DBS positive results should be confirmed using a different sample, e.g., fibroblasts, muscle, or GAA Sanger gene sequencing (13).
Leucocytes
GAA activity in mixed leucocytes from whole blood samples is a minimally invasive method that can be easily performed (26). The shipment of the sample needs to be fast because of the drop in enzyme activity during time. The use of acarbose to eliminate the interference by maltase glucoamylase (MGA) improves the reliability of this method.
GAA activity can be also measured in purified lymphocytes because isoenzymes [i.e., maltase-glucoamylase (MGA)] are absent in these cells (27). This method could still give some false-negative data if the sample is contamination with neutrophils. However, this method is rarely applied.
Fibroblasts
GAA activity can be measured in cultured skin fibroblasts with quite reliable results. Cultured fibroblasts required a skin biopsy that is a mini invasive procedure, but the enzyme assay can be performed only after growing fibroblasts. This can take up to 4–6 weeks with a significant delay in the diagnosis. For this reason, nowadays, this method is not considered as a first choice diagnostic method but rather as a confirmatory test after a DBS positive test (13).
Quantification of lymphocytes with glycogen-filled vacuoles in blood smear [blood smear examination (BSE)]
In Pompe disease, there is a widespread glycogen accumulation in all tissues and also in blood cells. Some years ago, it was observed that glycogen accumulation was present in lymphocytes that presented vacuolization on blood smear examination (BSE) (16).
This finding was confirmed over the years by others showing an increase in the number of vacuolated lymphocytes in LOPD patients compared to controls; the authors proposed to use blood smear examination as a possible diagnostic screening procedure (17). A recent study demonstrated that evaluation of vacuolated lymphocytes in BSE is a powerful test in the differential diagnosis of autophagic myopathies (18). More recently we confirmed that all vacuoles are periodic acid Schiff (PAS)-positive and that PAS stain is the preferred method to detect glycogen storage in LOPD patients’ lymphocytes (Figure 1). The presence of vacuolated lymphocytes seems very specific for Pompe disease. The number of PAS-positive lymphocytes in treated and untreated patients is quite different; although a small number of patients were studied, the difference was significant, suggesting that quantification of vacuolated lymphocytes on BSE can be considered a possible biomarker in therapeutic trials. These data suggest that BSE could be performed as a blood-based method in screening programs even before GAA assay in DBS which is a more sophisticated technique and not available in all diagnostic laboratories (19). However, BSE results need to be confirmed by the detection of enzyme deficiency and/or genetic analysis.
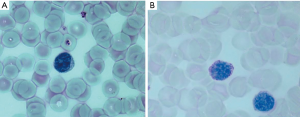
Muscle biopsy
In LOPD, muscle biopsy is still an important tool, but it should not be the first option for diagnostic purposes. Furthermore, different muscle groups and even fibers within the same muscle may show high variability in terms of muscle damage, the number of vacuolated fibers or glycogen storage.
This heterogeneity makes it difficult to establish a correlation between the degree of muscle damage and clinical status. In other words, the findings from a single biopsy site may be inconclusive.
Morphological aspects
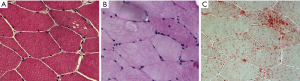
Histochemical studies of muscle biopsy may reveal a vacuolization of myofibers with glycogen accumulation inside the vacuoles but also free in the cytoplasm. Glycogen-filled vacuoles stain strongly for PAS (Figure 2). Acid phosphatase histochemistry reveals positive staining in all vacuolated fibers, indicating lysosomal dysfunction (20). Muscle fibers may show a widespread accumulation of autofluorescent lipofuscin inclusions resulting from inefficient lysosomal degradation (28). Unspecific changes such as fiber size variability, centralized nuclei, single fiber necrosis, basophilia and phagocytosis can be observed. Glycogen storage and fiber vacuolization in LOPD are less pronounced than in the infantile form of the disease, and the absence of pathology cannot rule out the diagnosis of Pompe disease (Figure 3). It is, however, important to emphasize that muscle biopsy can be very informative in differential diagnosis with other muscle disorders that can mimic Pompe disease (29). Moreover, muscle morphological studies are very important for understanding the pathogenesis of Pompe disease and the mechanisms of skeletal muscle damage. Few studies have focused on this issue reporting that ERT positively modifies skeletal muscle pathology with evident reduction of PAS-staining and vacuolated fibers in post-treatment biopsies, suggesting the efficacy of ERT in the clearance of lysosomal glycogen (30).
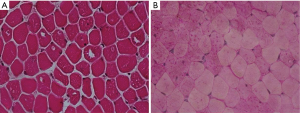
Muscle GAA assay
Muscle GAA assay is a reliable test to detect GAA deficiency. The values over 35% of reference ranges are not suggestive of Pompe disease. A reduction of GAA activity in fibroblasts or muscle below 30% is consistent with the LOPD diagnosis (2).
Genetic analysis
In the LOPD diagnostic algorithm, GAA analysis is recommended as confirmatory test in DBS positive patients; the genetic analysis is also useful for the identification of carriers in families with a history of LOPD.
GAA gene is located on 17q25 and spans 20 Kb. It contains 20 exons, and the start codon is at nucleotide 33 in exon 2 (31). Pompe Disease Mutation Database (http:/www.pompecenter.nl) is an updated source that provides all reported variants throughout the whole gene. More than 500 variants are reported and among them, 374 are pathogenic. Some mutations occur in specific populations but most of them are private. In distinct sub-populations, low enzyme activity has been related to a condition of pseudodeficiency due to the c.1726G>A (p.G576S) mutation of α-glucosidase (GAA) (32). This mutation has been found in the Asian population, namely in Japan and Taiwan. Most juvenile and adult Caucasian Pompe disease patients harbor the so called “leaky splice” mutation c.-32-13T>G in intron 1 of the gene (named as IVS1-13T>G) (33,34) in combination with another mutation on the second allele. This splice site mutation results in alternatively spliced transcripts, including a deletion of the first coding exon 2, but low amount of normal mRNA is still produced leading to some residual enzyme activity. Therefore, patients usually develop less severe form with late onset (35). Only few IVS1-13T>G homozygous patients have been described suggesting that individuals homozygous for this mutation may remain asymptomatic. A possible explanation is that modifying factors can influence the onset and the course of the disease (36). Recently we reported the phenotype and biochemical findings of six adult patients with Pompe disease demonstrating that homozygosity for c.-32-13T>G reflects the full adult Pompe disease phenotype spectrum (37).
The clinical presentation in compound heterozygous for IVS1 mutation is quite heterogeneous, reinforcing the notion that modifying factors can modulate the phenotype. Few studies have focused on this issue. Angiotensin-converting enzyme (ACE) I/D polymorphism and alpha actinin 3 (ACTN3) p.R577X polymorphism seem to be linked to the disease onset (38). A very recent study, looking for cis-acting single nucleotide variants (SNVs) in GAA gene, has identified the c.510C>T as a genetic modifier of the disease onset in compound heterozygous and homozygous IVS1 patients. The c.510 C>T reduced leaky wild-type splicing and has been associated with clinical manifestations of the disease in homozygotes and an earlier onset in the heterozygotes, although some additional genetic modifiers have been suggested (36).
The increased and early use of next generation sequencing (NGS)-based gene panels as diagnostic tool can be extended for the detection of GAA mutations because the gene is included in several commercially available NGS panels for muscle disorders, thus, facilitating the diagnosis even in atypical cases (39).
Final observations
Pompe disease is a progressive disorder and although there is an increased awareness among the clinicians, its diagnosis is still a challenge and is often delayed, especially in LOPD. ERT is fairly effective, but an early diagnosis and early initiation of therapy are important to obtain better therapeutic responses (40-42). A diagnostic protocol using BSE for identification of abnormal cytoplasmic vacuolation of lymphocytes and DBS to detect reduced GAA activity can be used as fast first-tier screening tests in LOPD high-risk populations followed by biochemical and genetic analysis leading to a timely and accurate diagnosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: In the last 3 years, A Toscano has received reimbursements from Sanofi Genzyme for teaching courses and as a member of Global Pompe Registry committee. O Musumeci received reimbursement for participation in invited lectures by Sanofi Genzyme.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: Acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, et al. (Eds.) The Metabolic & Molecular Bases of Inherited Disease, McGraw-Hill, New York, 2001:3389-420.
- van der Ploeg AT, Reuser AJ. Pompe's disease. Lancet 2008;372:1342-53. [Crossref] [PubMed]
- Toscano A, Musumeci O. Pathophysiological mechanisms in Glycogenosis type II. In: Filosto M, Toscano A, Padovani A, editors. Advances in Diagnosis and Management of Glycogenosis II. New York: Nova Science Publisher Inc; 2012:17-21.
- Chan J, Desai AK, Kazi ZB, et al. The emerging phenotype of late-onset Pompe disease: A systematic literature review. Mol Genet Metab 2017;120:163-72. [Crossref] [PubMed]
- Montagnese F, Barca E, Musumeci O, et al. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 2015;262:968-78. [Crossref] [PubMed]
- van Capelle CI, van der Meijden JC, van den Hout JM, et al. Childhood Pompe disease: clinical spectrum and genotype in 31 patients. Orphanet J Rare Dis 2016;11:65. [Crossref] [PubMed]
- Preisler N, Lukacs Z, Vinge L, et al. Late-onset Pompe disease is prevalent in unclassified limb-girdle muscular dystrophies. Mol Genet Metab 2013;110:287-9. [Crossref] [PubMed]
- Savarese M, Di Fruscio G, Torella A, et al. The genetic basis of undiagnosed muscular dystrophies and myopathies: Results from 504 patients. Neurology 2016;87:71-6. [Crossref] [PubMed]
- Angelini C, Semplicini C, Ravaglia S, et al. Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 2012;259:952-8. [Crossref] [PubMed]
- Chien YH, Hwu WL, Lee NC. Pompe disease: early diagnosis and early treatment make a difference. Pediatr Neonatol 2013;54:219-27. [Crossref] [PubMed]
- van der Ploeg AT, Kruijshaar ME, Toscano A, et al. European Pompe Consortium European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: a 10-year experience. Eur J Neurol 2017;24:768-e31. [Crossref] [PubMed]
- Wagner M, Chaouch A, Müller JS, et al. Presymptomatic late-onset Pompe disease identified by the dried blood spot test. Neuromuscul Disord 2013;23:89-92. [Crossref] [PubMed]
- Musumeci O, la Marca G, Spada M, et al. LOPED study: looking for an early diagnosis in a late-onset Pompe disease high-risk population. J Neurol Neurosurg Psychiatry 2016;87:5-11. [PubMed]
- Spada M, Porta F, Vercelli L, et al. Screening for later-onset Pompe's disease in patients with paucisymptomatic hyperCKemia. Mol Genet Metab 2013;109:171-3. [Crossref] [PubMed]
- Lukacs Z, Nieves Cobos P, Wenninger S, et al. Prevalence of Pompe disease in 3,076 patients with hyperCKemia and limb-girdle muscular weakness. Neurology 2016;87:295-8. [Crossref] [PubMed]
- Anderson G, Smith VV, Malone M, et al. Blood film examination for vacuolated lymphocytes in the diagnosis of metabolic disorders; retrospective experience of more than 2500 cases from a single centre. J Clin Pathol 2005;58:1305-310. [Crossref] [PubMed]
- Hagemans MLC, Stigter RL, van Capelle CI, et al. PAS-positive lymphocyte vacuoles can be used as diagnostic screening test for Pompe disease J Inherit Metab Dis 2010;33:133-9. [Crossref] [PubMed]
- Pascarella A, Terracciano C, Farina O, et al. Vacuolated PAS-positive lymphocytes as a hallmark of Pompe disease and other myopathies related to impaired autophagy. J Cell Physiol 2018;233:5829-37. [Crossref] [PubMed]
- Parisi D, Musumeci O, Mondello S, et al. Vacuolated PAS-Positive Lymphocytes on Blood Smear: An Easy Screening Tool and a Possible Biomarker for Monitoring Therapeutic Responses in Late Onset Pompe Disease (LOPD). Front Neurol 2018;9:880. [Crossref] [PubMed]
- Schoser BGH, Muller-Hockert L, Horvath R, et al. Adult-onset glycogen storage disease type 2: clinicopathological phenotype revisited. Neuropathol Appl Neurobiol 2007;33:544-59. [PubMed]
- Gaeta M, Musumeci O, Mondello S, et al. Clinical and pathophysiological clues of respiratory dysfunction in late-onset Pompe disease: New insights from a comparative study by MRI and respiratory function assessment. Neuromuscul Disord 2015;25:852-8. [Crossref] [PubMed]
- Musumeci O, Marino S, Granata F, et al. Central nervous system involvement in late-onset Pompe disease: clues from neuroimaging and neuropsychological analysis. Eur J Neurol 2019;26:442-e35. [Crossref] [PubMed]
- Musumeci O, Catalano N, Barca E, et al. Auditory system involvement in late onset Pompe disease: a study of 20 Italian patients. Mol Genet Metab 2012;107:480-4. [Crossref] [PubMed]
- Müller-Felber W, Horvath R, Gempel K, et al. Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord 2007;17:698-706. [Crossref] [PubMed]
- Kassardjian CD, Engel AG, Sorenson EJ. Electromyographic findings in 37 patients with adult-onset acid maltase deficiency. Muscle Nerve 2015;51:759-61. [Crossref] [PubMed]
- Kishnani PS, Amartino HM, Lindberg C, et al. Methods of diagnosis of patients with Pompe disease: Data from the Pompe Registry. Mol Genet Metab 2014;113:84-91. [Crossref] [PubMed]
- Winchester B, Bali D, Bodamer OA, et al. Methods for a prompt and reliable laboratory diagnosis of Pompe disease: Report from an international consensus meeting. Mol Genet Metab 2008;93:275-81. [Crossref] [PubMed]
- Feeney EJ, Austin S, Chien YH, et al. The value of muscle biopsies in Pompe disease: identifying lipofuscin inclusions in juvenile- and adult-onset patients. Acta Neuropathol Commun 2014;2:2. [Crossref] [PubMed]
- Vissing J, Lukacs Z, Straub V. Diagnosis of Pompe disease: muscle biopsy vs blood-based assays. JAMA Neurol 2013;70:923-7. [Crossref] [PubMed]
- Ripolone M, Violano R, Ronchi D, et al. Effects of short-to-long term enzyme replacement therapy (ERT) on skeletal muscle tissue in late onset Pompe disease (LOPD). Neuropathol Appl Neurobiol 2018;44:449-62. [Crossref] [PubMed]
- Hoefsloot LH, Hoogeveen-Westerveld M, Reuser AJ, et al. Characterization of the human lysosomal alphaglucosidase gene. Biochem J 1990;272:493-7. [Crossref] [PubMed]
- Tajima Y, Matsuzawa F, Aikawa S, et al. Structural and biochemical studies on Pompe disease and a "pseudodeficiency of acid alpha-glucosidase". J Hum Genet 2007;52:898-906. [Crossref] [PubMed]
- Huie ML, Chen AS, Tsujino S, et al. Aberrant splicing in adult onset glycogen storage disease type IIGSDII): molecular identification of an IVS1 (-13T->G) mutation in a majority of patients and a novel IVS10 (1GT->CT) mutation. Hum Mol Genet 1994;3:2231-6. [Crossref] [PubMed]
- Raben N, Nichols RC, Martiniuk F, et al. A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II). Hum Mol Genet 1996;5:995-1000. [Crossref] [PubMed]
- Kroos MA, Pomponio RJ, Hagemans ML, et al. Broad spectrum of Pompe disease in patients with the same c.-32-13T->G haplotype. Neurology 2007;68:110-5. [Crossref] [PubMed]
- Bergsma AJ, In 't Groen SLM, van den Dorpel JJA, et al. A genetic modifier of symptom onset in Pompe disease. EBioMedicine 2019;43:553-61.
- Musumeci O, Thieme A, Claeys KG, et al. Homozygosity for the common GAA gene splice site mutation c.-32-13T>G in Pompe disease is associated with the classical adult phenotypical spectrum. Neuromuscul Disord 2015;25:719-24. [Crossref] [PubMed]
- De Filippi P, Saeidi K, Ravaglia S, et al. Genotype-phenotype correlation in Pompe disease, a step forward. Orphanet J Rare Dis 2014;9:102. [Crossref] [PubMed]
- Savarese M, Torella A, Musumeci O, et al. Targeted gene panel screening is an effective tool to identify undiagnosed late onset Pompe disease. Neuromuscul Disord 2018;28:586-91. [Crossref] [PubMed]
- Toscano A, Montagnese F, Musumeci O. Early is better? A new algorithm for early diagnosis in late onset Pompe disease (LOPD). Acta Myol 2013;32:78-81. [PubMed]
- Toscano A, Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol 2013;260:951-9. [Crossref] [PubMed]
- Schoser B, Stewart A, Kanters S, et al. Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol 2017;264:621-30. [Crossref] [PubMed]


