
Variable clinical features and genotype-phenotype correlations in 18 patients with late-onset Pompe disease
Introduction
Glycogen storage disease type II (GSD), also known as Pompe disease (OMIM #232300), was first described by the Dutch pathologist Joannes Cassianus Pompe in 1932 (1). It is a rare, autosomal recessive disease with an overall reported population frequency ranging from approximately 1 in 40,000 to 1 in 57,000 (2,3). Pompe disease is caused by a deficiency in the lysosomal enzyme α-glucosidase (GAA) which causes pathologic glycogen accumulation in all tissues. This glycogen excess leads to multisystemic degenerative effects, most frequently affecting cardiac muscle, proximal limb muscles and diaphragm which causes earlier demise due to respiratory failure (4). Pompe disease has a wide variety of phenotypic expression with considerable overlap with other neuromuscular diseases, making diagnosis often a challenge (5). Newborn screening studies in several states however have addressed this delay in diagnosis and have indicated a significantly higher incidence of Pompe disease. Pompe disease can be associated with infantile onset and late onset myopathy, the phenotype sometimes correlating with the specific pathogenic variant in the GAA gene located at 17q25.3, and resulting residual enzyme level (6). Patients with the most severe form of Pompe disease, classic-infantile Pompe disease, have onset of symptoms within the first few weeks to months of life, with progressive cardiac hypertrophy typically leading to cardiorespiratory failure and death between 1–2 years of age (7-9). In other patients, onset of symptoms, including a slower progressive skeletal muscle weakness that affects mobility and respiratory function, presents ≤12 months of age without cardiac involvement or later in life during early childhood through late adulthood, sometimes as late as the sixth decade of life (10-14). Definitive diagnosis is made by molecular testing of the GAA gene and testing of enzymatic activity from blood or tissue (15).
Enzyme replacement therapy (ERT) utilizing recombinant human α-glucosidase (rhGAA) became commercially available in 2006 and has been used to help stabilize or improve patient outcomes (16-18). Early trials that included infantile patients demonstrated that ERT was safe, effective, and generally well-tolerated. Infants showed a reduction in cardiac hypertrophy as well as an improvement in cardiac and pulmonary function, muscle motility, and strength (19,20). In the late onset patients, several studies have shown an improvement in the muscle function measurements and pulmonary function studies compared to the natural history progression of untreated Pompe patients (21-26).
Methods
Patients
The study comprises of 18 adult patients with Pompe disease all of whom had documented deficiency of acid alpha-glucosidase enzyme activity and pathogenic variants in the GAA gene confirmed through molecular testing. Informed consent was obtained from all patients to use data from clinical evaluations performed and from their medical records for this natural history study. An overview of the patient demographics is provided (Table 1) and details of each patient are provided in Table S1.

Full table

Full table
Currently, all patients are receiving enzyme replacement therapy with alglucosidase alfa and are evaluated at six-month intervals by a medical geneticist, neuromuscular neurologist, genetic counselor, nutritionist, physical therapist, respiratory therapist, social worker, and a representative from the Muscular Dystrophy Association.
During the clinical visits, the following information was gathered: (I) demographics; (II) presence of specific clinical features including aneurysm, tinnitus, scoliosis, and osteoporosis; (III) ambulatory status and the type of assistive device being used if applicable; (IV) motor function—six-minute walk test (6MWT) (27), MRC scale and dynamometry; (V) respiratory status—the type of ventilatory support required; (VI) pulmonary function—forced vital capacity (FVC) both in upright seated and supine position, maximum inspiratory pressure (MIP), and sniff nasal inspiratory pressure (SNIP).
6MWT
Motor function was measured through the 6MWT according to the American Thoracic Society guidelines (27) in 16 patients. The distance covered by the patients were recorded in meters. Two patients (patient 5 and patient 12) were unable to do the 6MWT due to their partial or non-ambulatory status.
Pulmonary function test and respiratory muscle strength
Pulmonary function testing was measured in upright and supine positions in accordance with the ATS/European Respiratory Society guidelines (28). Percentages of the predicted FVC for both upright and supine positions were recorded. MIP and SNIP were recorded in cmH2O.
Statistical analyses
Descriptive statistics were provided for all categorical data, including patients’ gender and the associated clinical features seen in our cohort. For continuous variables (i.e., age), summary statistics (mean, median, range) were calculated. Multivariate linear mixed effect model was used to assess association between time and outcome measurements before and after treatment initiation. Random effect for each subject was included in the model to account for correlation of repeated measures within each patient. For measurements FVC upright and FVC supine, interaction of before vs. after treatment and time from ERT was examined to see whether patients’ outcome changes at different rate before and after treatment. However, for 6MWT, MIP, and SNIP, pre-treatment slope could not be examined because of the limited number of measurements in the pre-treatment period (less or equal to 3 patients had more than 2 time points at pre-treatment period). As a result, only post-treatment time trend was assessed for these measurements. Age at ERT initiation was adjusted as covariate in all models. Because there were five outcomes in this analysis, false discovery rate (FDR) P value adjustment was used to account for multiple comparisons.
GAA analysis
To confirm diagnosis, GAA sequencing for GSD II (Pompe disease) was done for all patients through peripheral blood sample. All our patients had done their genetic testing at Duke University Health System (DUHS) Molecular Diagnostics Laboratory or Emory Healthcare Laboratory Services by standard methodology (29). Also, changes to protein function are predicted based on algorithms that predict the effect of amino acid changes on protein structure and function based on the alignment of similar protein motifs (PolyPhen and SIFT).
Results
Group description
The gender, race, and age at varying time points throughout the course of the disease for the 18 patients are summarized in Table 1. The population was 77.78% (14/18) male and 22.22% (4/18) female, 17 patients are of Caucasian origin, including one mixed Chinese-Caucasian, and one patient is of Filipino descent. At the end of data collection, our cohort of patients had a mean age of 53.72 years (SD ±14.09), ranging from 22 to 74 years. For all patients, a history of progressive muscle disease of several years’ duration preceded their diagnosis at a mean age of 43.61 years (SD ±15.82), ranging from 11 to 65 years. The mean age at ERT initiation was 47.00 years (SD ±14.35), ranging from 12 to 66 years. Also, our cohort had a mean lag time of 14.06 years (SD ±9.31), ranging from 2 to 32 years, between their onset of symptoms to their confirmatory Pompe disease diagnosis. Patients were followed up to 10.7 years. after treatment initiation; the pre-treatment data (over a period of up to 22.4 years) were available for a few individuals.
Disease manifestations
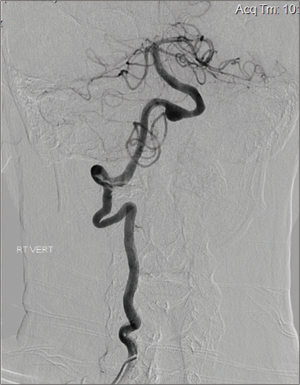
Table 1 provides a summary of the main demographic and clinical features and Table 2 provides the genotypes of all 18 patients in the study. Detailed clinical features of each individual patient are provided in Table S1. Among our cohort of patients, 94.4% (17/18) are currently ambulatory with 50% (9/18) not needing any assistive devices (cane/walker/wheelchair). Additionally, 12 use non-invasive respiratory support (66.6%), specifically BiPaP and one required tracheostomy and intermittent ventilation. A young adult with childhood onset and two adults [3/18 (16.7%)] had developed cardiomyopathy which improved with ERT. Hearing loss was present in 7/18 patients (38.9%) four of whom had tinnitus. Additional features seen were scoliosis in 5 (27.8%) and osteoporosis in 3 individuals (16.7%). Endocrinological features included hypothyroidism in 3 patients (16.7%); one patient (Patient 13) had both Graves’ disease and testicular cancer. Patient 2 at 42 years of age developed a cerebral aneurysm, which was treated with a Penumbra coil placement with no further incidents (Figure 1). Peripheral neuropathy was seen only in Patient 12 (5.6%).
Full table
Infusion reaction
Patient 9 (1/18, 5.6%) with the novel c.2655_2656delCG mutation experienced severe infusion reactions approximately 5 years after initiating ERT. In order to continue with her treatment, she took a regimen of 20 mg of famotidine (Pepcid) twice daily and 180 mg of the antihistamine fexofenadine (Allegra) on the day before, the day of and the day after her infusions. Additionally, she was premedicated with 20 mg dexamethasone (Decadron) and 50 mg diphenhydramine (Benadryl) and ranitidine HCl (Zantac) 50 mg intravenously. Elevations in her antibody titer (1/12,800) coincided with this reaction with subsequent improvement in the antibody titers as she was eventually able to immunotolerate her ERT with no further reactions.
GAA mutations
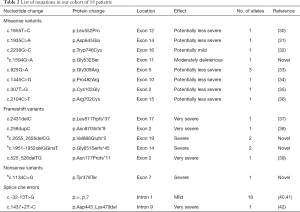
Our cohort of 18 Pompe patients include 3 sets of sibships in 14 unrelated families including: Patients 5, 6, & 7, Patients 9 & 10, and Patients 11 & 12. Genetic sequencing revealed that all 18 patients are compound heterozygotes for the GAA gene variants (Table S1, Table 2, Figure 2). The 36 alleles from our 18 patients consisted of 17 splice site variants (17/36, 47.2%), 10 missense variants (10/36, 27.8%), 8 frameshift variants (8/36, 22.2%), and 1 nonsense variant (1/36, 2.8%). By far, the most common variant observed was the common splicing variant c.-32-13T>G detected in 12/14 (85.7%) families. All the other mutations occurred in individual patients in this cohort.
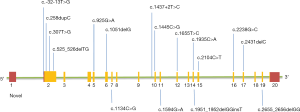
Thirteen of the seventeen GAA gene variants have previously been reported as pathogenic (http://www.pompecenter.nl). The four variants: c.1594G>A, c.2655_2656delCG, c.1951-1952delGGinsT, and c.1134C>G are novel. Variants c.2655_2656delCG, seen in siblings 9 & 10, and c.1951-1952delGGinsT, seen in siblings 11 & 12 are both indel (insertion/deletion) variants resulting in a premature termination codon, and thus a truncated protein product. Variant c.1134C>G seen in Patient 15 results in a stop codon in exon 7. Variant c.1594G>A, seen in Patient 2 was determined to be deleterious to protein function by PolyPhen and MutationTaster.
Respiratory function studies
FVC: upright seated and supine
Unfortunately, even with detailed ascertainment of medical records for each patient, data prior to the start of ERT were unavailable for the majority of patients. For those individuals for whom data were available, FVC upright before the start of ERT showed a significant decline with an estimated slope of –2.07 per year compared to predicted values (P<0.0001). In contrast, for all data points after the start of ERT, a slope of –0.17 was observed (P=0.23). The difference between these slopes, before and after start of ERT, was found to be highly significant (P<0.0001) (Figure 3A).
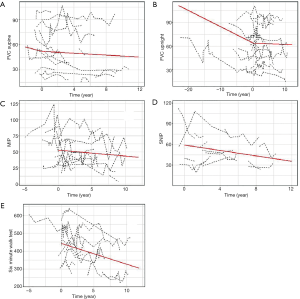
For FVC supine, the estimated decline before ERT was −2.67% per year (P=0.23), while the estimated decline of FVC supine after ERT was −0.55% per year (P=0.047). Although a −2.12 difference was observed in the slopes before and after ERT, this difference did not reach statistical significance (P=0.3656) (Figure 3B).
MIP and SNIP
For measures of MIP and SNIP, only slopes after ERT were examined. Post ERT, patients’ MIP declined at 0.92 per year (P=0.0169) and SNIP declined at 1.93 per year (P=0.0226). Figure 3C,D show the details of the fitted line respectively.
6MWT
While limited data on the 6MWT was available prior to ERT, deterioration in the slope post ERT was seen—the patients’ 6MWT was declining by 11.6 m per year (P<0.0001) as indicated by the detailed fitted line after ERT initiation (Figure 3E).
Summary of results of clinical findings
The mean age at diagnosis was 43.6 years (SD ±15.8), ranging from 11 to 65 years, and a mean lag time before diagnosis was 14.06 years (SD ±9.31). The mean age at ERT initiation was 47 years (SD ±14.35), ranging from 12 to 66. The most common pathogenic variant observed was c.-32-13T>G splicing variant detected in 16/36 alleles (44.4%) in 16/18 compound heterozygotes in 14 families. We report four novel mutations in trans with the c.-32-13T>G splicing variant associated with late onset Pompe disease. In our cohort 3/18 (16.7%) developed cardiomyopathy which improved with ERT. One male with the novel mutation c.1594 G>A had a cerebral aneurysm at the age of 42 years. Scoliosis was seen in 5/18 of the patients (28%). Tinnitus and hearing loss were noted in seven individuals (38.9% respectively). BiPAP is being used by 12 individuals (66.7%), additionally one individual had a tracheostomy and was ventilator and wheelchair dependent. One female with a severe infusion reaction and high antibody titers was treated successfully with a desensitization regimen (5.6%). One male had testicular cancer that was responsive to chemotherapy and Graves’ disease.
Discussion
This study reports the clinical variations and genotypes in 18 adult Pompe patients, and response to the initiation of ERT over a period of 25–90 months. We found that the mean age at diagnosis was 43.61 years with a mean diagnostic delay/lag time interval of 14.06 years. On average, patients in our cohort were diagnosed at a later stage than those reported in other studies (12,43). This delay, however, is now being addressed by newborn screening in many states.
The prevalence of scoliosis in our cohort (5/18, 27.78%) is comparable to the 24.8% (87/251) (44) and 23% (22/94) (45) found in other studies.
A higher incidence of the use of walking aids (9/18, 50%) was observed in our cohort compared to the incidences of 15% (14/94) (45), 38% (23/60) (25) and 54.5% (12/22) (46) reported in other studies. Similarly, we also observed higher incidence of respiratory support use in our patients (13/18, 72.2%) compared to that reported in other studies, 29% (27/94) (45), 33% (23/60) (25) and 40.1% (9/22) (46).
We report mild cardiomyopathy in 3/18 patients (16.7%), 2 having the common c.-32-13T>G variant. Our observation supports a recent study noting that severe cardiomyopathy is rare in patients with late-onset Pompe disease especially with the common c.-32-13T>G variant (47); all three patients showed improvement with ERT indicating that unlike the skeletal muscle, cardiac muscle is responsive to ERT. Nevertheless, we recommend regular surveillance for the cardiac manifestations of Pompe disease because of the 17% incidence of cardiomyopathy in our population.
We report one patient with a cerebral aneurysm at 42 years, which was successfully treated with a Penumbra coil placement. Cerebral arteries have been previously found to be affected by Pompe disease, and cerebral aneurysms are a complication. Subarachnoid hemorrhage secondary to rupture of the MCA aneurysm was reported in a 50-year-old man with late-onset Pompe disease. The patient was admitted for an acute onset of severe occipital thunderclap headache. His aneurysm was successfully clipped, and the patient had no neurological deficit on discharge (48). On review of the literature four late-onset Pompe patients with the c.-32-13T>G allele were reported with basilar artery dolichoecstasia or internal carotid dilatative arteriopathy (49). In a recent study, 16/23 infantile patients (70%) had ventricular enlargement and/or extra-axial cerebrospinal fluid accumulation at baseline neuroimaging; delayed myelination was detected in two of them. Follow-up neuroimaging (n=8) after 6–153 months showed marked improvement, with normalization of the baseline changes (50). However, two of three patients imaged after age 10 years demonstrated white matter changes, and one was noted to have a basilar artery aneurysm (50). More recently 13/21 patients (62%) showed intracranial arterial abnormalities of whom: 9.5% showed an unruptured intracranial aneurysm and 47% had a vertebrobasilar dolichoectasia (12). We also recommend surveillance by regular neuroimaging using computed tomography angiography (CTA) or magnetic resonance angiography (MRA) for cerebrovascular malformations in order to avoid sub-arachnoid hemorrhages.
We report one patient who was diagnosed with testicular cancer in his teens who was treated with excision and chemotherapy. We are aware of one other young male with Pompe syndrome with testicular cancer who has also responded well to standard treatment (personal communication with VK). To our knowledge, the association with testicular cancer has not been previously reported. Interestingly, a Pompe patient has been reported with thymic neuroendocrine carcinoma (51) and another patient, treated with ERT, was reported with a benign muscle lingual pseudohypertrophy (52). While the extent of the relationship between Pompe disease and the risk for malignancies is unknown, another lysosomal storage disorder, Gaucher disease, has long been associated with cancer, specifically multiple myeloma and lymphoma (53,54). Larger studies may determine if there is an increased risk for testicular malignancy or other types of malignancies in Pompe disease.
The most common c.-32-13T>G variant was seen in 16/36 (44.44%) alleles, in 17/18 (94.44%) patients and 12/14 (85.71%) families in our study cohort. It is well documented that c.-32-13T>G is the most common pathogenic variant identified in the GAA gene in Caucasian patients with late onset Pompe disease. The T>G transversion within intron 1 alters mRNA splicing resulting in the formation of alternative mRNA transcripts lacking exon 2. The altered splicing site is leaky with a small fraction of the normal RNA transcripts produced resulting in the late onset Pompe phenotype (55,56).
We also identified 17 unique GAA variants including four novel variants (3 in trans with the common mutation c.-32-13T>G) not previously reported in the literature in association with Pompe disease. The novel missense mutation c.1594G>A (p.Gly532Ser) is found in the only Asian patient from the Philippines in our cohort who also had a cerebral aneurysm. Recently, an additional patient has been identified with the same variant through newborn screening in 2016. This patient was a 3-week-old African-American male identified to have this variant along with the common splicing variant c.-32-13T>G (personal communication with VK).
Two patients with the novel frameshift mutations c.2655_2656delCG had high antibody titers and one of them had an adverse event to ERT that was treated successfully with medication. The patient with novel variant c.1951-1952delGGinstT had peripheral neuropathy, whereas the patient with another novel variant c.1134C>G did not have any atypical features.
Since data prior to the initiation of ERT was limited, a comparison of the slopes pre ERT and post ERT was challenging. In our most complete parameter studied, upright FVC showed significant improvement in the patients’ decline after starting ERT (−2.07 per year before ERT to −0.17 per year after starting ERT, P<0.0001). A similar difference is observed in supine FVC showing that patients declined −2.67 per year before ERT to −0.55 per year after starting ERT (P=0.047). This positive response to ERT especially in the rates of FVCs upright and supine was also noted by other authors (25,26). However, we noticed continued gradual decline in our patients’ 6MWT post ERT which may be partly attributed to the normal aging process. On the other hand, this decline may reflect limited efficacy of the ERT on muscle strength in contrast to the striking benefits on cardiac function. This was also reported by Kuperus who noted that distance walked on the 6MWT increased during the initial years of treatment, followed by a gradual decline. Compared to baseline, the median 6MWT distance increased from 376 to 416 m at 5 years of treatment and compared to baseline, 69% of patients improved their walking ability at 5 years of treatment (26).
Our patient cohort illustrates significant variability in the range of clinical features and alerts us to the importance of careful monitoring and early management of these complications. Possible genotype-phenotype associations with the novel variants identified may emerge with larger studies.
Acknowledgments
We received funding for the Pompe registry by Sanofi-Genzyme, 50 Binney Street Cambridge, MA 02142.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from all patients to use data from clinical evaluations performed and from their medical records for this natural history study.
References
- Pompe JC. Over idiopatische hypertrophie van het hart. Ned Tijdschr Geneeskd 1932;76:304.
- Martiniuk F, Chen A, Mack A, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease. Am J Med Genet 1998;79:69-72. [Crossref] [PubMed]
- Ausems MG, Verbiest J, Hermans MP, et al. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur J Hum Genet 1999;7:713-6. [Crossref] [PubMed]
- Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. The metabolic and molecular bases of inherited disease. 2001;3:3389-420.
- Al-Lozi MT, Amato AA, Barohn RJ, et al. Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve 2009;40:149-60. [Crossref] [PubMed]
- Engel AG, Seybold ME, Lambert EH, et al. Acid maltase deficiency: comparison of infantile, childhood, and adult types. Neurology 1970;20:382. [PubMed]
- van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003;112:332-40. [Crossref] [PubMed]
- Kishnani PS, Hwu WL, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 2006;148:671-6. [Crossref] [PubMed]
- Reuser AJJ, Hirschhorn R, Kroos MA. Pompe Disease: Glycogen Storage Disease Type II, Acid α-Glucosidase (Acid Maltase) Deficiency. In: Beaudet AL, Vogelstein B, Kinzler KW, et al. editors. The Online Metabolic and Molecular Bases of Inherited Disease. New York, NY: The McGraw-Hill Companies, Inc.; 2014.
- Winkel LP, Hagemans ML, van Doorn PA, et al. The natural course of non-classic Pompe's disease; a review of 225 published cases. J Neurol 2005;252:875-84. [Crossref] [PubMed]
- Kishnani PS, Howell RR. Pompe disease in infants and children. J Pediatr 2004;144:S35-43. [Crossref] [PubMed]
- Montagnese F, Barca E, Musumeci O, et al. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 2015;262:968-78. [Crossref] [PubMed]
- Reuser AJ. Enzyme therapy in Pompe disease: questions remain. Mol Genet Metab 2012;107:243-author reply 4. [Crossref] [PubMed]
- Kanters TA, van der Ploeg AT, Kruijshaar ME, et al. Cost-effectiveness of enzyme replacement therapy with alglucosidase alfa in adult patients with Pompe disease. Orphanet J Rare Dis 2017;12:179. [Crossref] [PubMed]
- Amato AA. Acid maltase deficiency and related myopathies. Neurol Clin 2000;18:151-65. [Crossref] [PubMed]
- Chien YH, Lee NC, Huang HJ, et al. Later-Onset Pompe Disease: Early Detection and Early Treatment Initiation Enabled by Newborn Screening. J Pediatr 2011;158:1023-7.e1. [Crossref] [PubMed]
- Cupler EJ, Berger KI, Leshner RT, et al. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve 2012;45:319-33. [Crossref] [PubMed]
- Merk T, Wibmer T, Schumann C, et al. Glycogen storage disease type II (Pompe disease)–influence of enzyme replacement therapy in adults. Eur J Neurol 2009;16:274-7. [Crossref] [PubMed]
- Klinge L, Straub V, Neudorf U, et al. Enzyme replacement therapy in classical infantile pompe disease: results of a ten-month follow-up study. Neuropediatrics 2005;36:6-11. [Crossref] [PubMed]
- Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26-33. [Crossref] [PubMed]
- Regnery C, Kornblum C, Hanisch F, et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J Inherit Metab Dis 2012;35:837-45. [Crossref] [PubMed]
- Strothotte S, Strigl-Pill N, Grunert B, et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol 2010;257:91-7. [Crossref] [PubMed]
- Bembi B, Pisa F, Confalonieri M, et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J Inherit Metab Dis 2010;33:727-35. [Crossref] [PubMed]
- Angelini C, Semplicini C, Ravaglia S, et al. Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 2012;259:952-8. [Crossref] [PubMed]
- van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med 2010;362:1396-406. [Crossref] [PubMed]
- Kuperus E, Kruijshaar ME, Wens SCA, et al. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology 2017;89:2365-73. [Crossref] [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002;166:518-624. [Crossref] [PubMed]
- Goldstein JL, Dickerson G, Kishnani P, et al. Blood‐based diagnostic testing for Pompe disease: Consistency between GAA enzyme activity in dried blood spots and GAA gene sequencing results. Muscle Nerve 2014;49:775-6. [Crossref] [PubMed]
- Bodamer OA, Haas D, Hermans MM, et al. L-alanine supplementation in late infantile glycogen storage disease type II. Pediatr Neurol 2002;27:145-6. [Crossref] [PubMed]
- Hermans MM, de Graaff E, Kroos MA, et al. The conservative substitution Asp-645-->Glu in lysosomal alpha-glucosidase affects transport and phosphorylation of the enzyme in an adult patient with glycogen-storage disease type II. Biochem J 1993;289:687-93. [Crossref] [PubMed]
- Wan L, Lee CC, Hsu CM, et al. Identification of eight novel mutations of the acid α-glucosidase gene causing the infantile or juvenile form of glycogen storage disease type II. J Neurol 2008;255:831-8. [Crossref]
- Kroos MA, van Leenen D, Verbiest J, et al. Glycogen storage disease type II: identification of a dinucleotide deletion and a common missense mutation in the lysosomal alpha-glucosidase gene. Clin Genet 1998;53:379-82. [Crossref] [PubMed]
- Kroos M, Hoogeveen-Westerveld M, Michelakakis H, et al. Update of the pompe disease mutation database with 60 novel GAA sequence variants and additional studies on the functional effect of 34 previously reported variants. Hum Mutat 2012;33:1161-5. [Crossref] [PubMed]
- Hermans MM, van Leenen D, Kroos MA, et al. Twenty-two novel mutations in the lysosomal alpha-glucosidase gene (GAA) underscore the genotype-phenotype correlation in glycogen storage disease type II. Hum Mutat 2004;23:47-56. [Crossref] [PubMed]
- Montalvo AL, Cariati R, Deganuto M, et al. Glycogenosis type II: identification and expression of three novel mutations in the acid alpha-glucosidase gene causing the infantile form of the disease. Mol Genet Metab 2004;81:203-8. [Crossref] [PubMed]
- Liu X, Wang Z, Jin W, et al. Clinical and GAA gene mutation analysis in mainland Chinese patients with late-onset Pompe disease: identifying c.2238G > C as the most common mutation. BMC Med Genet 2014;15:141. [Crossref] [PubMed]
- Beesley CE, Child AH, Yacoub MH. The identification of five novel mutations in the lysosomal acid a-(1-4) glucosidase gene from patients with glycogen storage disease type II. Mutations in brief no. 134. Online. Hum Mutat 1998;11:413. [Crossref] [PubMed]
- Bali DS, Goldstein JL, Banugaria S, et al. Predicting cross-reactive immunological material (CRIM) status in Pompe disease using GAA mutations: lessons learned from 10 years of clinical laboratory testing experience. Am J Med Genet C Semin Med Genet 2012;160C:40-9. [Crossref] [PubMed]
- Herzog A, Hartung R, Reuser AJ, et al. A cross-sectional single-centre study on the spectrum of Pompe disease, German patients: molecular analysis of the GAA gene, manifestation and genotype-phenotype correlations. Orphanet J Rare Dis 2012;7:35. [Crossref] [PubMed]
- Huie ML, Chen AS, Tsujino S, et al. Aberrant splicing in adult onset glycogen storage disease type II (GSDII): molecular identification of an IVS1 (-13T-->G) mutation in a majority of patients and a novel IVS10 (+1GT-->CT) mutation. Hum Mol Genet 1994;3:2231-6. [Crossref] [PubMed]
- Stroppiano M, Bonuccelli G, Corsolini F, et al. Aberrant splicing at catalytic site as cause of infantile onset glycogen storage disease type II (GSDII): molecular identification of a novel IVS9 (+2GT-->GC) in combination with rare IVS10 (+1GT-->CT). Am J Med Genet 2001;101:55-8. [Crossref] [PubMed]
- Remiche G, Ronchi D, Magri F, et al. Extended phenotype description and new molecular findings in late onset glycogen storage disease type II: a northern Italy population study and review of the literature. J Neurol 2014;261:83-97. [Crossref] [PubMed]
- Roberts M, Kishnani PS, van der Ploeg AT, et al. The prevalence and impact of scoliosis in Pompe disease: Lessons learned from the Pompe Registry. Mol Genet Metab 2011;104:574-82. [Crossref] [PubMed]
- van der Beek NA, de Vries JM, Hagemans ML, et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis 2012;7:88. [Crossref] [PubMed]
- Stepien KM, Hendriksz CJ, Roberts M, et al. Observational clinical study of 22 adult-onset Pompe disease patients undergoing enzyme replacement therapy over 5 years. Mol Genet Metab 2016;117:413-8. [Crossref] [PubMed]
- Herbert M, Cope H, Li JS, et al. Severe Cardiac Involvement Is Rare in Patients with Late-Onset Pompe Disease and the Common c.-32-13T>G Variant: Implications for Newborn Screening. J Pediatr 2018;198:308-12. [Crossref] [PubMed]
- Peric S, Fumic K, Bilic K, et al. Rupture of the middle cerebral artery aneurysm as a presenting symptom of late-onset Pompe disease in an adult with a novel GAA gene mutation. Acta Neurol Belg 2014;114:165-6. [Crossref] [PubMed]
- Sacconi S, Bocquet J, Chanalet S, et al. Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol 2010;257:1730-3. [Crossref] [PubMed]
- McIntosh PT, Hobson-Webb LD, Kazi ZB, et al. Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy. Mol Genet Metab 2018;123:85-91. [Crossref] [PubMed]
- Aruj PK, Rausch S, De Vito EL. Thymic neuroendocrine carcinoma with Pompe's disease of the adult. Medicina (B Aires) 2015;75:315-8. [PubMed]
- Milisenda JC, Pujol T, Grau JM. Not only bright tongue sign in Pompe disease. Neurology 2016;87:1629-30. [Crossref] [PubMed]
- Rosenbloom BE, Weinreb NJ, Zimran A, et al. Gaucher disease and cancer incidence: a study from the Gaucher Registry. Blood 2005;105:4569-72. [Crossref] [PubMed]
- Zimran A, Liphshitz I, Barchana M, et al. Incidence of malignancies among patients with type I Gaucher disease from a single referral clinic. Blood Cells Mol Dis 2005;34:197-200. [Crossref] [PubMed]
- Kroos M, Hoogeveen-Westerveld M, Michelakakis H, et al. Update of the pompe disease mutation database with 60 novel GAA sequence variants and additional studies on the functional effect of 34 previously reported variants. Hum Mutat 2012;33:1161-5. [Crossref] [PubMed]
- Laforêt P, Nicolino M, Eymard B, et al. Juvenile and adult-onset acid maltase deficiency in France: Genotype-phenotype correlation. Neurology 2000;55:1122-8. [Crossref] [PubMed]


