Clinical features and pregnancy outcomes of women with abnormal cell-free fetal DNA test results
Introduction
Cell-free fetal DNA (cffDNA) is fetal DNA circulating freely in the maternal blood. Due to the length differences, cffDNA can be distinguished from maternal DNA fragments. Analysis of cffDNA may provide earlier diagnosis of fetal conditions, especially fetal chromosomal diseases. Noninvasive prenatal testing (NIPT) is a novel prenatal screening method, which involves detection of fetal cffDNA in maternal plasma via massively parallel sequencing (MPS). This method has been widely used in prenatal screening for trisomies 21 (T21), 18 (T18), and 13 (T13). Many studies have confirmed that it yields good accuracy and a low false positive rate (1-3). Due to its lack of invasiveness, NIPT is readily accepted by pregnant women.
Beginning in 2012, many international organizations have issued relevant expert consensus or guidelines regarding NIPT. In 2015, the American College of Obstetricians and Gynecologists (ACOG) (4) updated its consensus and recommended that NIPT should be provided for prenatal screening of fetal aneuploidy. However, traditional prenatal screening programs are still applied first. The International Society for Prenatal Diagnosis (ISPD) (5) suggested that NIPT could be used in women at intermediate risk after serological prenatal screening to reduce the chances of a missed diagnosis. The American College of Medical Genetics (ACMG) (6) suggested that NIPT could replace traditional serological prenatal screening. In China, perinatal medical experts also tend to use NIPT as a second-line prenatal screening procedure.
NIPT has been widely applied in prenatal screening for T21, T18, and T13 due to its high accuracy and sensitivity. Its detection rates (DR) and false positive rates (FPR) are reportedly 99.7% and 0.04% for T21, 97.9% and 0.04% for T18, and 99.0% and 0.04% for T13, respectively (2). NIPT can also be used to detect other fetal genetic diseases, such as fetal sex chromosome aneuploidy (7). In our study, the total positive predictive value (PPV) of NIPT was 54.54%, which was 29.41% for Turner syndrome, 77.78% for 47,XXY, and 100% for 47,XXX and 47,XYY (7). Several studies have suggested that the use of NIPT is beneficial in the context of screening for microdeletions/microduplications, but that its detection efficiency is not satisfactory, e.g., low PPV (8).
NIPT is well understood and accepted by increasing numbers of pregnant women. However, there is still a lack of acceptance of NIPT in some countries or regions. It is necessary to make steps toward distinguishing between prenatal screening and prenatal diagnosis, determine how to reasonably decide on further prenatal diagnosis, and how to make the decision to continue or terminate pregnancy. Many factors affect these decisions, including the type of fetal disease, the level of prenatal genetic counseling, the quality of life of the pregnant woman, cultural factors, multiple cross-disciplinary approaches, etc. For example, one study reported that pregnancy was continued in 66% of women with fetal SCA counseled by a perinatologist, while only 36% of such women continued their pregnancies if they were counseled by a general obstetrician (9). Other studies have reported that the rate of pregnancy termination was reduced significantly after the establishment of multidisciplinary centers for prenatal diagnosis (MCPD) (10,11).
In the present study, we retrospectively analyzed the clinical data of 228 pregnant women with positive NIPT results and examined their clinical treatments, results of prenatal diagnosis, and pregnancy outcomes. The goal was to identify the factors affecting the selection of clinical treatment among these women to improve prenatal genetic counseling.
Methods
Patients and design
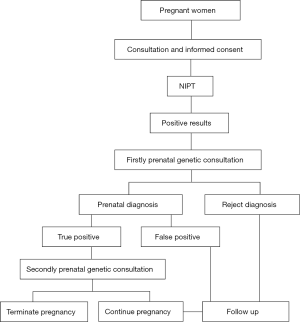
From January 2012 to December 2017, a total of 228 women received positive NIPT results at our institute. The positive results mainly included high risks of T21, T18, T13, and SCA. The women were 16–49 years old and their gestational weeks were 12+4–27+0 w. Table 1 lists the demographic characteristics of these women. Figure 1 presents an outline of the flow of prenatal diagnosis and clinical treatment after an abnormal NIPT result. The study design and protocol were reviewed and approved by the ethics committee of Changzhou Maternity and Child Health Care Hospital, affiliated with Nanjing Medical University (No. 201501).

Full table
Prenatal diagnosis and clinical treatment
On receiving a positive NIPT result, a short message was sent immediately to the pregnant woman and she was recalled by the specialist. After recall, the pregnant women received prenatal genetic consultation by a professional geneticist. If the women accepted the prenatal diagnosis, they received amniocentesis at a suitable gestational stage (18–23 weeks). Prenatal diagnosis was carried out according to our routine experimental method, and was completed in our prenatal diagnosis center. NIPT was performed on the Illumina Next CN 500 sequencing platform (1,7). The SNP array test was performed using a commercial 750K microarray chip (Affymetrix CytoScan 50K Array) (12). Cytogenetic prenatal diagnoses were similar to our previous reports (1,13,14). Based on the NIPT results, we performed chromosomal microarray analysis (CMA) for prenatal diagnosis in some of the pregnant women. The accuracy of NIPT was determined based on the results of fetal chromosomal karyotyping and CMA. The women with true positive results received prenatal genetic counseling again, and decided whether to continue or terminate the pregnancy according to the specific circumstances. Women who continued the pregnancy or who had false positive results on NIPT were specifically advised regarding the importance of prenatal ultrasound check and their pregnancy outcomes were followed.
Statistical analysis
The data were analyzed using EmpowerStats software (X&Y Solutions, Boston, MA) and R (http://www.R-project.org) (15). The χ2 test was used to compare differences in continuous variables between two groups. The risk of chromosomal abnormalities was estimated by univariate analysis. In all analyses, P<0.05 was taken to indicate statistical significance.
Results
Within the study period, a total of 17,894 pregnant women underwent NIPT examinations at our institute and 228 received positive results, including 125 for common fetal aneuploidies (91 as T21, 28 as T18, 6 as T13), 95 for fetal sex chromosome aneuploidies (56 as Turner syndrome, 21 as Klinefelter syndrome, 12 as XXX syndrome, 6 as XYY syndrome), and 8 for microdeletion or microduplication involving multiple autosomes or sex chromosomes. Among the 228 cases with positive results, advanced age (age ≥35 years) (77, 33.8%), high risk after prenatal screening (risk of T21>1/300 or T18 >1/350) (76, 33.3%), and intermediate risk (risk of T21, T18 between high risk and 1/1,000) (33, 16.2%) were the three most common groups. The mean gestational week when the women underwent NIPT was 17+3 weeks.
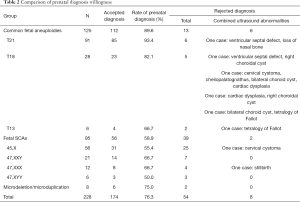
Women received the results within 3–8 days after sample collection. Those with positive test results were recalled by telephone within a median of 1 day (range 1–3 days) after the results were determined. All 228 women were successfully recalled (rate of 100%). After prenatal genetic counseling, 174 women (76.3%) accepted the prenatal diagnosis, and 54 women (23.7%) rejected the diagnosis for various reasons, such as severe ultrasound abnormalities, worry about abortion, etc. In comparison to the women with positive results for SCA, the rate of prenatal diagnosis for women with positive results of common fetal aneuploidies was significantly higher (58.9% vs. 89.6%, respectively, P<0.05), as shown in Table 2 and Figure 2. It should be noted that among the 13 women with positive NIPT results for T21/T18/T13 who rejected diagnosis, severe ultrasonic abnormalities were observed in six cases (Table 2). Although we did not perform fetal genetic diagnosis in these cases, but these ultrasound abnormalities were suggested to be closely related to the chromosomal anomalies.
Full table
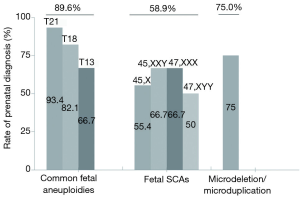
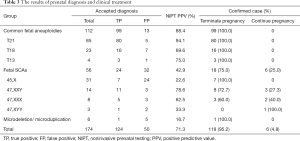
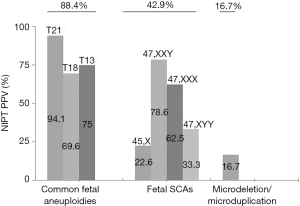
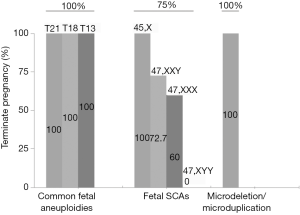
Among the 174 women who accepted the prenatal diagnosis, all received the results within 3 weeks. After prenatal diagnosis, 124 women were confirmed to have true positive results. The PPV of T21/T18/T13 and SCAs were 88.4% and 42.9%, respectively (Table 3). The PPV of T21 was highest (94.1%), while that of Turner syndrome was lowest (22.6%) (Figure 3). After receiving the results of prenatal diagnosis, 100% of women received further genetic counseling and 96.8% women made a decision within 3 days. After the second prenatal genetic counseling, a total of 118 (95.2%) women with true positive results selected to terminate their pregnancy. All of the women carrying fetuses with T21/T18/T13 terminated their pregnancy (99/99, 100%). One quarter (6/24, 25.0%) of the women with SCA continued their pregnancy (Table 3, Figure 4). Women carrying fetuses with Turner syndrome were more willing to terminate their pregnancy.
Full table
We followed the pregnancy outcomes of three groups. Seven women selected to continue their pregnancy after diagnosis as true positive; they all delivered and complications developed in two cases (one case of intestinal adhesion and one case of neonatal anemia). In total, 50 women were confirmed to have false positive results; 49 of them had effective follow-up results and all delivered live infants. One male infant had hypospadia and one case was lost to follow-up. Among the 54 women who rejected prenatal diagnosis, 53 were successfully followed up and one was lost to follow-up. Of these, 42 women delivered live infants, but neonatal karyotype analysis was not performed; 7 showed severe abnormalities on ultrasound, which were closely related to the chromosomal diseases in late pregnancy, and they selected to terminate their pregnancies. Another 4 women had a spontaneous abortion or stillbirth.
In total, 8 women were shown to have microdeletion or microduplication by NIPT. Of them, 6 accepted prenatal diagnosis by CMA and one was confirmed as true positive: NIPT showed Chr(9) microduplication in this woman. After prenatal diagnosis, the fetal chromosomal karyotype was 46,XN,add9(33), and the CMA result was 9q31.1q33.1(107,923,508-121,624,320)*3. A database query did not clarify the significance of the copy number variations (CNV). This woman chose to continue her pregnancy, and she delivered a male infant at term; he was treated for intestinal adhesion.
Discussion
NIPT currently focuses on three target conditions: trisomy 21 (T21), trisomy 18 (T18), and trisomy 13 (T13). In the present study, these three conditions accounted for almost 54.8% of all positive NIPT results. After prenatal genetic counseling, about 90% of women were willing to accept the prenatal diagnosis. Among the 10% of women who rejected diagnosis, more than half showed different fetal ultrasound abnormalities that were closely related to the chromosomal diseases. After prenatal diagnosis, the PPV of T21/T18/T13 was 88.4%. The women with true positive results made the decision immediately, and all of the women selected to terminate their pregnancy. The above results showed that NIPT had good accuracy for detection of T21/T18/T13, particularly T21. The compliance of pregnant women was better because they know more about these three conditions. Due to the seriousness of the disease, most pregnant women are willing to undergo prenatal diagnosis and almost all of the positive cases will terminate their pregnancy in China. These three diseases account for half of the positive NIPT results. Therefore, NIPT should not be used alone to detect these three diseases.
Fetal SCA accounted for a large proportion (41.7%) of the positive results. Similar to previous reports (2,7,16), we confirmed that NIPT could also be used to detect fetal SCA. The PPV of SCA was 42.9% in this study. There were significant differences in PPV between different types of SCA diseases, with the lowest for Turner syndrome, similar to our previous report (7). There is no consensus regarding whether routine prenatal screening for fetal SCA should be performed, and it is generally regarded as an adjunct to NIPT. However, some groups have reported that children with prenatal diagnosis have a milder developmental course than children ascertained postnatally, especially with regard to mental health and social adaptation (17,18). In comparison to the women with positive results for T21/T18/T13, the rate of prenatal diagnosis for women with positive SCA results was significantly reduced. Even with a diagnosis of fetal SCA, a substantial proportion of pregnant women were willing to continue their pregnancy. There were also significant differences in the rates of pregnancy termination between different types of SCA disease. Women carrying fetuses with Turner syndrome were more willing to terminate their pregnancy, while women with other fetal SCAs tended to continue their pregnancy. There is no consensus regarding clinical treatment after prenatal diagnosis of fetal SCA. Gruchy et al. (10) performed a series of studies focusing on this issue. They reported that with regard to Turner syndrome, the rate of pregnancy termination was closely related to the 45,X karyotype, mosaic karyotype, and structural abnormalities of the X chromosome. With the opening of multidisciplinary prenatal diagnosis centers (MPDCs) in France, the rate has dropped from 90.4% to 79.6% (10). The termination rates for 47,XXX and 47,XYY also fell significantly after 1997 (from 41.1% to 11.8% and from 25.8% to 6.7%, respectively) following the implementation of MPDCs in France (19). In the present study, the rates of pregnancy termination were higher those reported by Gruchy et al. Almost all women with fetuses positive for Turner syndrome selected to terminate their pregnancy, although there were no cases of mosaic karyotype in our cohort. This may have been related to the level of prenatal genetic counseling, the cognition level of pregnant women, economic situation, cultural factors, etc.
Our results also suggested that NIPT may also be useful for identifying submicroscopic chromosomal abnormalities. The PPV of the current conventional NIPT scheme was only 16.7%, although expansion of NIPT to include microdeletion syndromes has been controversial and inclusion of other imbalances that can be detected through NIPT has not been widely discussed (8,20). However, accumulating evidence indicates that technically optimized NIPT is effective for identifying fetal substructural chromosomal abnormalities, with reported detection rates of 97.8% for 22q11.2 deletion and 100% for Prader-Willi, Angelman, 1p36 deletion, and cri-du-chat syndromes (21). However, identification of microdeletions was associated with lower PPV and higher false positive rates (22). We do believe that this will be improved markedly with optimization of the NIPT scheme.
There are very strict restrictions and regulations for termination of pregnancy in China, especially in late pregnancy. To terminate a pregnancy over 14 weeks of gestation, it is generally necessary to go to a designated prenatal diagnostic institution and discuss it with doctors. Multidisciplinary consultation is usually required for termination of a pregnancy over 28 weeks.
This study had some limitations. First, we did not perform neonatal karyotype analysis in women who rejected prenatal diagnosis. Therefore, we could not determine whether the NIPT test results were accurate, especially for fetal SCA. It is well known that patients with sex chromosome aneuploidy are phenotypically normal in the neonatal stage without physical or intellectual disability, and it is difficult to identify SCA syndrome without karyotype analysis before adolescence (7). Although we transferred confirmed cases with continuation of pregnancy to our department of pediatrics, effective rehabilitation remains a topic to be discussed in future.
In conclusion, we retrospectively analyzed 228 pregnant women with positive NIPT results, and systematically investigated the accuracy of NIPT detection and subsequent clinical treatment. NIPT showed good detection accuracy for T21/T18/T13, and also contributed to identification of fetal SCA and substructural chromosomal abnormalities. When given a positive NIPT result, the attitudes of pregnant women regarding prenatal diagnosis and clinical treatment are related to the severity of the disease, cognitive ability, and the level of prenatal genetic counseling.
Acknowledgments
We thank all of the project participants for their contributions. We also thank Textcheck (http://www.textcheck.com) for language editing.
Funding: This study was supported by grants from projects supported by the National Natural Science Foundation of China (81773438): Key Research and Development Plan Project of Jiangsu Province (BE2017650), Jiangsu Maternal and Child Health Research Project (F201754), Changzhou Science and Technology Support Project (Social Development CE20175021), and Changzhou Applied Basic Research Program (CJ20179033).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study design and protocol were reviewed and approved by the ethics committee of Changzhou Maternity and Child Health Care Hospital, affiliated with Nanjing Medical University (No. 201501). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Yu B, Lu BY, Zhang B, et al. Overall evaluation of the clinical value of prenatal screening for fetal-free DNA in maternal blood. Medicine (Baltimore) 2017;96:e7114. [Crossref] [PubMed]
- Gil MM, Accurti V, Santacruz B, et al. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2017;50:302-14. [Crossref] [PubMed]
- Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG 2017;124:32-46. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists. Cell-free DNA screening for fetal aneuploidy. Committee Opinion No. 640. Obstet Gynecol 2015;126:e31-7. [Crossref] [PubMed]
- Benn P, Borell A, Chiu R, et al. Position statement from the Aneuploidy Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn 2013;33:622-9. [Crossref] [PubMed]
- Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genetics in Medicine 2016;18:1056-65. [Crossref] [PubMed]
- Zhang B, Lu BY, Yu B, et al. Noninvasive prenatal screening for fetal common sex chromosome aneuploidies from maternal blood. J Int Med Res 2017;45:621-30. [Crossref] [PubMed]
- Advani HV, Barrett AN, Evans MI, et al. Challenges in non-invasive prenatal screening for sub-chromosomal copy number variations using cell-free DNA. Prenat Diagn 2017;37:1067-75. [Crossref] [PubMed]
- Shaw SW, Chueh HY, Chang SD, et al. Parental decisions regarding prenatally detected fetal sex chromosomal abnormality and the impact of genetic counselling: an analysis of 57 cases in Taiwan. Aust N Z J Obstet Gynaecol 2008;48:155-9. [Crossref] [PubMed]
- Gruchy N, Vialard F, Blondeel E, et al. Pregnancy outcomes of prenatally diagnosed Turner syndrome: a French multicenter retrospective study including a series of 975 cases. Prenat Diagn 2014;34:1133-8. [Crossref] [PubMed]
- Gruchy N, Vialard F, Decamp M, et al. Pregnancy outcomes in 188 French cases of prenatally diagnosed Klinefelter syndrome. Hum Reprod 2011;26:2570-5. [Crossref] [PubMed]
- Long W, Gu J, Ouyang J, et al. Genetic analysis of two fetuses with congenital heart defects and 3q microdeletion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2018;35:240-3. [PubMed]
- Yu B, Li H, Chen YP, et al. Clinical evaluation of NIPS for women at advanced maternal age: a multicenter retrospective study. J Matern Fetal Neonatal Med 2018.1-6. [Crossref] [PubMed]
- Chen YP, He ZQ, Shi Y, et al. Not all chromosome aberrations can be detected by NIPT in women at advanced maternal age: A multicenter retrospective study. Clin Chim Acta 2018;486:232-6. [Crossref] [PubMed]
- Wu J, Geng J, Liu L, et al. The Relationship between Estimated Glomerular Filtration Rate and Diabetic Retinopathy. J Ophthalmol 2015;2015:326209. [Crossref] [PubMed]
- Scibetta EW, Gaw SL, Rao RR, et al. Clinical accuracy of abnormal cell-free fetal DNA results for the sex chromosomes. Prenat Diagn 2017;37:1291-7. [Crossref] [PubMed]
- Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. Am J Med Genet 2002;110:11-8. [Crossref] [PubMed]
- Ross JL, Quigley CA, Cao D, et al. Growth hormone plus childhood low-dose estrogen in Turner's syndrome. N Engl J Med 2011;364:1230-42. [Crossref] [PubMed]
- Gruchy N, Blondeel E, Le Meur N, et al. Pregnancy outcomes in prenatally diagnosed 47, XXX and 47, XYY syndromes: a 30-year French, retrospective, multicentre study. Prenat Diagn 2016;36:523-9. [Crossref] [PubMed]
- Benn P. Expanding non-invasive prenatal testing beyond chromosomes 21, 18, 13, X and Y. Clin Genet 2016;90:477-85. [Crossref] [PubMed]
- Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol 2015;212:332.e1-9. [Crossref] [PubMed]
- Petersen AK, Cheung SW, Smith JL, et al. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am J Obstet Gynecol 2017;217:691.e1-691.e6. [Crossref] [PubMed]
- Godino L, Pompilii E, D'Anna F, et al. Attitudes of women of advanced maternal age undergoing invasive prenatal diagnosis and the impact of genetic counselling. Eur J Hum Genet 2016;24:331-7. [Crossref] [PubMed]