
Point of care ultrasound in thoracic malignancy
Introduction
Medical ultrasonography was first described in 1947 after Dussik successfully used intracranial ultrasound to detect tumors (1). Shortly after, ultrasound was adopted into several different fields including cardiology, obstetrics and radiology (2). In 1988, ultrasonography was described as the stethoscope of the future, though some physicians were concerned that the technology would not be used properly (3).
Ultrasonography is a safe, efficient and cost-effective mode of imaging that can assist clinicians with diagnostic and procedural guidance (4-6). Ultrasound technology has advanced quickly over the last two decades with small portable ultrasound transducers for less than $2,000, that can connect to a smart phone (2,7). Although often performed by technicians, ultrasound, when used by clinicians at point of care, whether in the intensive care unit (ICU) or outpatient clinic can provide important clinical data for immediate decision making, that would otherwise require more expensive traditional imaging including radiography or computed tomography (CT) scans (6). In addition to the financial benefit, bedside ultrasound has been shown to reduce complications in intravenous access, thoracentesis, lumbar puncture (LP) and paracentesis (5,8-13).
Today, point of care ultrasonography (POCUS) has become ubiquitous in most hospitals. Emergency medicine residency programs were the first programs mandated to provide POCUS training in 2009 and other specialties followed shortly after (14). Currently, POCUS is utilized in many specialties for inpatient and outpatient care.
Basics of ultrasonography
Proper use of POCUS requires understanding of not only the anatomy and pathophysiology, but also the physics of ultrasound. Diagnostic ultrasonography uses an electrical source to power piezoelectric crystals on the surface of the ultrasound transducer which converts the signal from electrical energy to soundwaves. As the sound waves penetrate the tissue, some are reflected depending on the interphase and density of the tissue. The reflected soundwaves are processed and converted to electric signals that is displayed as 2-dimentional grayscale image (15,16).
The selection of the correct transducer depends on the target location, field of view, tissue type, depth and intended mode of use (17). Most transducers create a soundwave frequency between 1–15 MHz which is well above the upper level of human hearing at 20 kHz (15). The lower frequency transducers provide better penetration at the expense of lower resolution and conversely, higher frequency transducers have better resolution with less penetration.
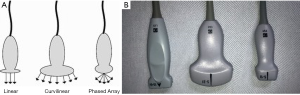
There are three common types of transducers for POCUS: linear, curvilinear and phased array (Figure 1). The linear transducer contains the piezoelectric crystals arranged in a line that are wired to function at the same time to construct a single image. Linear transducers are best used for evaluation of vasculature, muscle, tendon and other superficial structures because they operate at around 3–11 MHz. This frequency allows for better resolution at a shallow depth. The curvilinear probe also contains piezoelectric crystals arranged in a linear fashion, which are wired to operate simultaneously. However, the transducer shape is convex to allow for a larger pie-shaped field of view. The curvilinear probe is best used for abdominal structures because it operates at around 2–5 MHz which allows for better penetration of deeper structures. The phased array transducer operates in a different fashion than the linear and curvilinear transducers. The piezoelectric crystals of the phased array transducer are wired sequentially which causes a sweeping motion. The phased array transducer operates around 2–5 MHz which facilitates deeper penetration and the sweeping motion allows for the waves to penetrate through narrow areas like rib spaces while viewing cardiac, lung and pleural structures (15-18).
Diagnostic ultrasound is a safe, well tolerated mode of imaging. Unlike CT and radiography, ultrasound does not use ionizing radiation and will not increase lifetime risk of malignancy (19). The potential complications of ultrasound include thermal injury and mechanical effects. The thermal injury happens due to oscillating particles from the soundwaves heating surrounding tissue. The mechanical effects may result in small bubbles produced in the tissue by the ultrasound causing micro cavitation and potential damage to the surrounding tissue. The mechanical and thermal complications are most often seen with other ultrasound modes that use a higher intensity for therapeutic purposes. POCUS uses a lower intensity soundwave that has not been documented to cause significant or permanent damage (16).
Diagnostic role of POCUS in malignancy
Pleural effusion
Malignant pleural effusion (MPE) presents when there is metastatic spread of disease to the pleural space. POCUS allows clinicians to visualize pleural effusion earlier and help characterize their etiology more accurately than chest radiograph and CT scans. Pleural effusion up to 500 mL may not be seen on chest radiograph while a pleural effusion as small as 3-5mL may be seen with ultrasound (20). POCUS can help distinguish between transudative and exudative effusion in some cases. Exudative effusions frequently contain loculations or echogenic fluid but can also present as anechoic homogenous fluid on ultrasound (21). Transudative effusions typically present as anechoic although complex nonseptated imaging without homogeneous echogenic pattern can also be seen (22). Normal pleura is <2 mm thick on ultrasound and a thickness of 3 mm or more of the parietal pleura is more likely to suggest exudative effusion (23,24). Finally, metastatic hyper-echoic nodules may be detected on the diaphragm or parietal pleura which may be missed with conventional imaging (12,23).
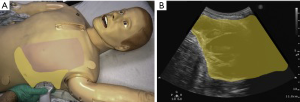
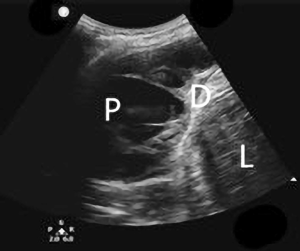
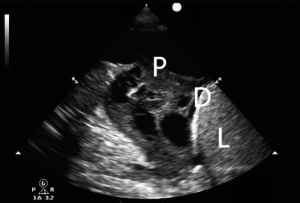
Although the optimal position of the patient for evaluation of pleural fluid is suggested to be supine (25), the ultrasound probe can be used while the patient is sitting by placing the curvilinear or the phased array transducer in the midaxillary line either in the longitudinal or transverse axis and sliding it inferiorly until the liver, diaphragm and lung can be visualized (Figure 2). A pleural effusion appears as an anechoic triangle above the diaphragm. The presence of fluid allows the ultrasound waves to pass through and may allow the lumbar vertebrae to be visualized. The presence of lumbar vertebrae on lung ultrasound is called the “spine sign” and is pathognomonic for pleural effusion (Figure 3) (26). We routinely use the ultrasound examination to determine if there is enough fluid accumulation before considering a thoracentesis. The benefit of ultrasound is the ability to detect pleural effusion in any position even at bedside, however, one must remember that the effusion tends to collect in the dependent area and as such the ultrasound probe may need to be positioned appropriately in the dependent area. For example, in a supine patient, a very small effusion may be detected if the ultrasound probe is held perpendicular to the gravitational pull. Actual accumulation of effusion can be detected by tilting or moving the ultrasound probe in the dependent location (Figure 4).

The advent of POCUS has made it easier to identify pleural effusion in the outpatient or inpatient setting and has improved the ability to safely drain them with fewer complications (13,27). We routinely evaluate all patients suspected of having an effusion in the outpatient clinic with an ultrasound examination to identify the amount and whether there is any loculation (Figures 5,6) (24).
Lung cancer
CT and positron emission tomography (PET) scans are the standard of care for diagnosis, initial staging and follow up of treatment response of lung cancer (28). Approximately 5% of all primary lung cancers and 45% of all T3 lung cancers invade the chest wall (29). CT and PET scans can be limited in identifying if a peripheral lung cancer is invading the chest wall (30). POCUS can be used in addition to CT and PET scans to help further characterize the tumor, obtain diagnostic tissue and to determine if the tumor is metastasizing to the visceral pleura, the parietal pleura, chest wall or liver for appropriate staging prior to surgery. Lung tumors confined to visceral pleura are classified as T2 which have a better prognosis than lung tumors that involve the visceral pleura into the parietal pleura which will be classified as T3. POCUS can help stage peripheral lung tumors appropriately to ensure the patient receives the proper treatment (30).
Pulmonary edema
Pulmonary edema in patients with cancer on chemotherapy could result from heart failure or other non-cardiogenic etiologies. Heart failure from anthracyclines, cyclophosphamide and high-dose external beam radiation can have insidious onset and may initially present as dyspnea on exertion (31). Non cardiogenic pulmonary edema has been seen following treatment with interleukin-2, gemcitabine and all-trans retinoic acid (32). POCUS allows for quick evaluation of pulmonary edema in an inpatient or office setting. POCUS can accurately differentiate pulmonary edema from other etiologies for acute dyspnea (Figure 7) (33,34).
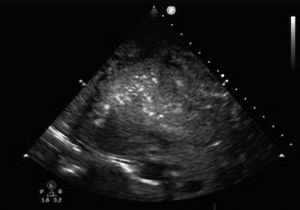
The curvilinear and phased array transducers are optimal for evaluating patients for pulmonary edema. The linear probe can be used for evaluating the anterior chest and pleura but, the deeper structures may be missed due to the high frequency of the ultrasound wave (25). Each lung should be evaluated in three different areas: the upper anterior point, the lower anterior point and the posterolateral point. POCUS of the lung relies on interpreting artifacts that are generated by the ultrasound waves interacting with air in the lung. A-lines are echogenic horizontal lines parallel to the pleural line and does not move with respiration; these represent normal aerated lungs. B-lines are defined by hyperechoic artifacts arising from the pleural line to the bottom of the screen that move with lung sliding and ablate the A-lines (35). B-lines develop when the ultrasound encounters an air-fluid interface and this represents an interstitial pattern that has a differential diagnosis of pulmonary edema, interstitial pneumonia, or diffuse parenchymal lung disease. Diffuse anterior B-lines in combination with the presence of lung sliding are 97% sensitive and 95% specific for pulmonary edema (36). B-lines that are asymmetric without lung sliding are consistent with a consolidation (35).
Ascites
Ascites is frequently caused by liver dysfunction, however, up to 10% of cases can be caused by a malignancy. Malignant ascites frequently occurs from tumors in the peritoneal cavity like the uterus, ovaries, colon and pancreas but can also occur from metastatic spread of breast, lung and lymphoma. Patients with malignant ascites have a poor prognosis with an average life expectancy of 20 weeks at the time of diagnosis (37). POCUS can be used to help characterize the ascitic fluid and assess for malignant characteristics.
A curvilinear transducer and a supine patient position are optimal to visualize ascites and the peritoneal cavity (17). Simple ascites is anechoic and echogenicity can be seen in fluid with a low serum ascites albumin gradient, hemorrhagic or malignant ascites. Features associated with malignant effusion are loculations, presence of liver adhesions and matted bowel loops. An abdominal ultrasound examination may reveal masses, metastatic liver nodules and lymphadenopathy, which could be indirect signs of malignant ascites (38). Diagnosing malignant ascites by imaging alone is very difficult and is confirmed with fluid sampling (39).
VTE
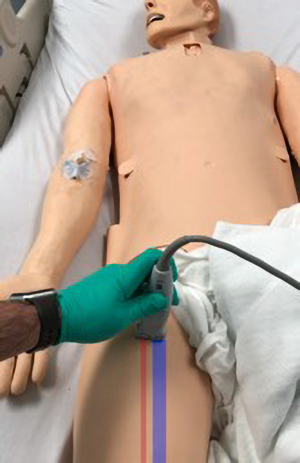
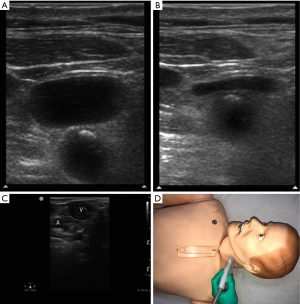
About 20% of all new VTE cases are related to underlying malignancy (40). POCUS is a fast and easy way to diagnose proximal deep vein thrombosis (DVT). Physicians with formal ultrasound training and <2-year experience have 95% accuracy in diagnosing proximal DVTs using the compression technique (41). The linear high frequency transducer is optimal for vascular structures. Extended compression ultrasound (ECUS) is the preferred protocol for assessing DVTs with POCUS (42). ECUS protocol involves starting with the leg externally rotated and evaluating the common femoral vein proximally.
The linear probe should be placed in the transverse plane so that the common femoral vein (CFV) and common femoral artery (CFA) can be visualized (Figure 8). The ultrasound transducer is used to compress the CFV and CFA. Under normal physiology, the CFV will collapse and the lumen will not be visible while the CFA maintains its structure and lumen. If a DVT is present, the CFV may collapse a little or not at all (43). If the DVT is acute, it will appear anechoic while chronic DVTs may be echogenic in the lumen of the vein (44). The probe should be placed on the proximal CFV and moved distally in 2 cm segments with intermittent compressions through the femoral and popliteal veins ending in the popliteal fossa. If the study is negative for DVT, a doppler ultrasound study should be obtained (42). Similarly the compression ultrasound can be used to locate clots in the neck vessels (Figure 9).
Metastatic disease
The presence of metastatic disease will change both treatment options and anticipated prognosis. The standard staging and surveillance of patients with malignancy is PET/CT scan, however, there may be benefits to utilizing POCUS. POCUS is portable, inexpensive and quick, which may allow for lung cancer surveillance especially in populations with limited resources.
Non-small cell lung cancer most commonly metastasizes to the adrenal glands or liver (45). Metastatic disease of the adrenal glands can be visualized with POCUS. However, metastatic adrenal lesions can vary in size, location and density and will need fine needle aspiration to diagnose metastatic disease (46). Liver metastases can be visualized with ultrasonography but sensitivity remains low at around 40%. Contrast enhanced ultrasonography can more than double the sensitivity which equates to that of CT (47). In addition to diagnosing new metastatic disease, contrast enhanced ultrasound can allow for evaluating response for liver metastases after therapy (48). The role for POCUS in diagnosing and monitoring metastatic lesions is not entirely clear and requires further evaluation.
Therapeutic role of POCUS
Ultrasound guided procedures
Thoracentesis, placement of pleural catheters, chest tubes or evaluation before pleuroscopy
There are two ultrasound techniques used during procedures, dynamic or real time technique and static or marking technique. The dynamic or real time technique refers to the physician using the ultrasound probe in real time to visualize and guide the needle to the pleural effusion. With a single operator, additional skills may be required to hold the probe in one hand and insert the needle with the other. Real time ultrasound has been shown to reduce pneumothorax, dry taps, and bleeding while improving the diagnostic yield in smaller effusion (13). In addition, real time ultrasound can be used to visualize vascular structures which can help a clinician avoid damage to multiple vessels including the intercostal artery and its branches during thoracentesis (49). The static technique refers to a technician or a physician first examining the pleural effusion and marking the location on the skin to guide needle placement. The thoracentesis is then completed using the marked site as a point of entry. One should be careful to insert the needle in the same axis and direction of the ultrasound probe when the site was marked. The static technique does not improve rate of complication compared to performing the procedure without any guidance and thus is not recommended over the real time technique (50,51). If the pleural effusion is malignant or recurrent, POCUS can also be utilized to guide insertion of small bore chest tubes and placement of tunneled pleural catheters. We use the ultrasound prior to pleuroscopy to locate the site of maximum pleural fluid accumulation.
Paracentesis
Paracentesis is generally well-tolerated and is frequently performed with ultrasound guidance. A serious potential complication of paracentesis is damage to the inferior epigastric artery which can result in hemorrhage or death (10). POCUS can assist in reducing the risk of complications by allowing to assess the location of the inferior epigastric artery prior to the procedure and by directly visualizing the peritoneal cavity during the performance of the procedure (9,10). The preferable location for paracentesis is in the right or left lower abdominal quadrants and ultrasound allows us to find a safe window away from any small bowel for diagnostic or therapeutic aspiration (Figure 10). Ultrasound guided paracentesis not only reduces complications but has been shown to have decreased financial burden on the health care system (13).
Vascular access
Vascular access in the patient with cancer can be difficult and frequently requires placement of a port for infusion and frequent blood testing (52). POCUS can be utilized to assist with vascular access if the patient does not have a port or if it is malfunctioning. POCUS has been well described and is now standard for placement of central venous catheters (CVC).
POCUS decreases complications like pneumothorax, arterial cannulation and bleeding, and improves success rate while decreasing overall cost (5,53). POCUS has transcended the intensive care setting and has shown benefit for placement of peripherally inserted central catheters (PICC) and peripheral IVs in an emergency department, inpatient setting or infusion center (52,54). POCUS allows for a vein to be evaluated prior to cannulation to observe patency, lumen dimensions and pathways to ensure that the optimal vessel is selected. Utilizing POCUS can potentially decrease the need to place CVCs in non-critical patients with difficult vascular access (55). Physicians, nurses and technicians can benefit from using POCUS while placing vascular access because it causes less complications, requires fewer attempts of the procedure and spares CVC placement in non-critical patients (5,53,56,57).
LP
Patients with malignancy may require a LP for diagnostic purposes or for therapeutic purposes including intrathecal chemotherapy. LP can be difficult on patients with an increased BMI, abnormal landmarks or variant anatomy (58). POCUS can be used to improve success and decrease complications. POCUS can be used to help provide a more detail imaging of the spine that cannot be interpreted on physical exam such as depth of the ligamentum flavum, calcifications or mild scoliosis prior to the LP. However, the use of static ultrasound prior to LP has not shown any benefit compared to the anatomy guided (blind) technique (59). Real time use of ultrasound during LP has been shown to have higher success rate compared to the blind technique and decreased traumatic punctures (60).
Conclusions
POCUS is a safe, cost-effective and easily implemented imaging modality that can assist with evaluation of dyspnea, pleural effusion, metastatic disease in the chest and abdomen, and identification of DVT. It has been shown to improve procedural efficiency while decreasing complications, increasing success and reducing financial strain on the health care system.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shampo MA, Kyle RA. Karl Theodore Dussik—Pioneer in Ultrasound. Mayo Clinic Proceedings 1995;70:1136. [Crossref] [PubMed]
- Krishnamoorthy VK, Sengupta PP, Gentile F, et al. History of echocardiography and its future applications in medicine. Crit Care Med 2007;35:S309-13. [Crossref] [PubMed]
- Filly RA. Ultrasound: the stethoscope of the future, alas. Radiology 1988;167:400. [Crossref] [PubMed]
- Alpert JS, Mladenovic J, Hellmann DB. Should a hand-carried ultrasound machine become standard equipment for every internist? Am J Med 2009;122:1-3. [Crossref] [PubMed]
- Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia 2004;59:1116-20. [Crossref] [PubMed]
- Peris A, Tutino L, Zagli G, et al. The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg 2010;111:687-92. [Crossref] [PubMed]
- "Butterfly Network 510(k) Premarket Notification FDA Approval." Retrieved 4/19/2018. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K163510.pdf
- Ferre RM, Sweeney TW. Emergency physicians can easily obtain ultrasound images of anatomical landmarks relevant to lumbar puncture. Am J Emerg Med 2007;25:291-6. [Crossref] [PubMed]
- Stone JC, Moak JH. Feasibility of sonographic localization of the inferior epigastric artery before ultrasound-guided paracentesis. Am J Emerg Med 2015;33:1795-8. [Crossref] [PubMed]
- Sekiguchi H, Suzuki J, Daniels CE. Making paracentesis safer: a proposal for the use of bedside abdominal and vascular ultrasonography to prevent a fatal complication. Chest 2013;143:1136-9. [Crossref] [PubMed]
- Bruzoni M, Slater BJ, Wall J, et al. A prospective randomized trial of ultrasound- vs landmark-guided central venous access in the pediatric population. J Am Coll Surg 2013;216:939-43. [Crossref] [PubMed]
- Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest 2011;140:1332-41. [Crossref] [PubMed]
- Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest 2013;143:532-8. [Crossref] [PubMed]
- Ahern M, Mallin MP, Weitzel S, et al. Variability in Ultrasound Education among Emergency Medicine Residencies. West J Emerg Med 2010;11:314-8. [PubMed]
- Lawrence JP. Physics and instrumentation of ultrasound. Crit Care Med 2007;35:S314-22. [Crossref] [PubMed]
- Martin DJ, Wells ITP, Goodwin CR. Physics of ultrasound. Anaesthesia & Intensive Care Medicine 2015;16:132-5. [Crossref]
- Szabo TL, Lewin PA. Ultrasound transducer selection in clinical imaging practice. J Ultrasound Med 2013;32:573-82. [Crossref] [PubMed]
- Shriki J. Ultrasound physics. Crit Care Clin 2014;30:1-24. v. [Crossref]
- Shankar H, Pagel PS. Potential adverse ultrasound-related biological effects: a critical review. Anesthesiology 2011;115:1109-24. [Crossref] [PubMed]
- Gryminski J, Krakówka P, Lypacewicz G. The Diagnosis of Pleural Effusion by Ultrasonic and Radiologic Techniques. Chest 1976;70:33-7. [Crossref] [PubMed]
- Sajadieh H, Afzali F, Sajadieh V, et al. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci 2004;1019:585-92. [Crossref] [PubMed]
- Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol 2008;34:362-9. [Crossref] [PubMed]
- Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992;159:29-33. [Crossref] [PubMed]
- Feller-Kopman D. Ultrasound-guided thoracentesis. Chest 2006;129:1709-14. [Crossref] [PubMed]
- Miller A. Practical approach to lung ultrasound. BJA Education 2016;16:39-45. [Crossref]
- Liu RB, Donroe JH, McNamara RL, et al. The Practice and Implications of Finding Fluid During Point-of-Care Ultrasonography: A Review. JAMA Intern Med 2017;177:1818-25. [Crossref] [PubMed]
- Barnes TW, Morgenthaler TI, Olson EJ, et al. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound 2005;33:442-6. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e50S.
- Filosso PL, Sandri A, Guerrera F, et al. Primary lung tumors invading the chest wall. J Thorac Dis 2016;8:S855-S62. [Crossref] [PubMed]
- Bandi V, Lunn W, Ernst A, et al. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest 2008;133:881-6. [Crossref] [PubMed]
- Stover DE, Kaner RJ. Pulmonary complications in cancer patients. CA Cancer J Clin 1996;46:303-20. [Crossref] [PubMed]
- Briasoulis E, Pavlidis N. Noncardiogenic pulmonary edema: an unusual and serious complication of anticancer therapy. Oncologist 2001;6:153-61. [Crossref] [PubMed]
- Miglioranza MH, Gargani L, Sant'Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141-51. [Crossref] [PubMed]
- Pivetta E, Goffi A, Lupia E, et al. Lung Ultrasound-Implemented Diagnosis of Acute Decompensated Heart Failure in the ED: A SIMEU Multicenter Study. Chest 2015;148:202-10. [Crossref] [PubMed]
- Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134:117-25. [Crossref] [PubMed]
- Dietrich CF, Mathis G, Blaivas M, et al. Lung B-line artefacts and their use. J Thorac Dis 2016;8:1356-65. [Crossref] [PubMed]
- Sangisetty SL, Miner TJ. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg 2012;4:87-95. [Crossref] [PubMed]
- Edell SL, Gefter WB. Ultrasonic differentiation of types of ascitic fluid. AJR Am J Roentgenol 1979;133:111-4. [Crossref] [PubMed]
- Smereczynski A, Kolaczyk K, Bernatowicz E. Difficulties in differentiating the nature of ascites based on ultrasound imaging. J Ultrason 2017;17:96-100. [Crossref] [PubMed]
- Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol 2009;22:9-23. [Crossref] [PubMed]
- Kory PD, Pellecchia CM, Shiloh AL, et al. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest 2011;139:538-42. [Crossref] [PubMed]
- Needleman L, Cronan JJ, Lilly MP, et al. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation 2018;137:1505-15. [Crossref] [PubMed]
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795-8. [Crossref] [PubMed]
- Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. CMAJ 2006;175:1087-92. [Crossref] [PubMed]
- Milovanovic IS, Stjepanovic M, Mitrovic D. Distribution patterns of the metastases of the lung carcinoma in relation to histological type of the primary tumor: An autopsy study. Ann Thorac Med 2017;12:191-8. [Crossref] [PubMed]
- Fan J, Tang J, Fang J, et al. Ultrasound imaging in the diagnosis of benign and suspicious adrenal lesions. Med Sci Monit 2014;20:2132-41. [Crossref] [PubMed]
- Wu W, Chen MH, Yin SS, et al. The Role of Contrast-Enhanced Sonography of Focal Liver Lesions Before Percutaneous Biopsy. AJR Am J Roentgenol 2006;187:752-61. [Crossref] [PubMed]
- Krix M, Plathow C, Essig M, et al. Monitoring of liver metastases after stereotactic radiotherapy using low-MI contrast-enhanced ultrasound--initial results. Eur Radiol 2005;15:677-84. [Crossref] [PubMed]
- Kanai M, Sekiguchi H. Avoiding Vessel Laceration in Thoracentesis: A Role of Vascular Ultrasound With Color Doppler. Chest 2015;147:e5-e7. [Crossref] [PubMed]
- Raptopoulos V, Davis LM, Lee G, et al. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol 1991;156:917-20. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii61-76. [Crossref] [PubMed]
- DeWitty RL, Siram SM, Balkissoon J. Vascular Access in the Cancer Patient. J Natl Med Assoc 1986;78:289-91. [PubMed]
- Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003;327:361. [Crossref] [PubMed]
- Bauman M, Braude D, Crandall C. Ultrasound-guidance vs. standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med 2009;27:135-40. [Crossref] [PubMed]
- Shokoohi H, Boniface K, McCarthy M, et al. Ultrasound-guided peripheral intravenous access program is associated with a marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann Emerg Med 2013;61:198-203. [Crossref] [PubMed]
- Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med 2005;23:363-7. [Crossref] [PubMed]
- Powell JT, Mink JT, Nomura JT, et al. Ultrasound-guidance can reduce adverse events during femoral central venous cannulation. J Emerg Med 2014;46:519-24. [Crossref] [PubMed]
- Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract 2016;6:358-68. [Crossref] [PubMed]
- Lahham S, Schmalbach P, Wilson SP, et al. Prospective evaluation of point-of-care ultrasound for pre-procedure identification of landmarks versus traditional palpation for lumbar puncture. World J Emerg Med 2016;7:173-7. [Crossref] [PubMed]
- Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ 2013;346:f1720. [Crossref] [PubMed]


