
A case of chemorefractory metastatic type AB thymoma sensitive to helical tomotherapy
Introduction
Type AB thymoma associated with multiple metastases is rarely seen in the clinic (1). Chemotherapy is the main treatment for these tumors, but most patients are clinically non-responsive to chemotherapy, so radiotherapy is often used for symptomatic relief (2). However, the therapeutic effect and feasibility of a new type of radiotherapy, helical tomotherapy (TOMO), have not yet been reported. In order to deepen the understanding of thymoma and to assess the use of TOMO in its treatment, we retrospectively reviewed patient medical records at the Department of Radiation Oncology, The First Affiliated Hospital of Bengbu Medical University, in addition to the available literature indexed in PubMed.
Case presentation
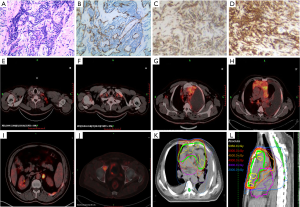
A male patient aged 52 with cough and expectoration was diagnosed with type AB thymoma by pathology and immunohistochemistry (Figure 1A,B,C,D). PET/CT indicated an anterior mediastinal mass, invasion of the pleura and pericardium associated with the right hip bone and systemic multiple lymphatic metastases (stage IVB) (Figure 1E,F,G,H,I,J).
After a definitive diagnosis, chemotherapy was administered for 2 cycles (CAP regimen, once every 3 weeks), as the primary therapy. The efficacy evaluation indicated PD, which prompted the use of IMRT (54 Gy/30 fractions) for primary lesion (Figure 1K,L). In the meantime, a chemotherapy regimen (DP regimen, once every 3 weeks) was used for 2 cycles during radiotherapy. After radiotherapy, a re-examination of PET-CT illustrated the obvious shrinkage of the anterosuperior mediastinal primary masses, without a significant increase in SUVmax in the residual masses; the areas that did not receive radiotherapy showed lesion enlargement (Figure 2A,B,C,D). The efficacy evaluation indicated PD under chemotherapy and PR in the radiotherapy area, so TOMO was used further.
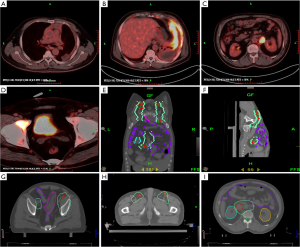
According to PET/CT imaging, the target area was delineated which included the bilateral groin, and retroperitoneal metastatic lesions (56 Gy/28 fractions) (Figure 2E,F,G,H,I). During TOMO radiotherapy, the pain of the patient was significantly relieved and showed a marked decrease in leukocytes and platelets, and the treatment was stopped at the 18th time because the number of leukocytes and platelets continued to decrease greatly. The patient asked to be discharged and died after 50 days. The overall survival time of the patient from disease diagnosis to death was about 10 months.
Discussion and conclusions
The incidence of thymoma accounts for 20% of all mediastinal tumors, and the incidence is about 1.5 parts per million per year. The peak age of thymoma onset is 40–50 years old, with the condition being predominant in males (3). This is because androgen can promote the proliferation and maturation of thymoma cells, whereas estrogen can inhibit their growth (4). Although it has been proven that at least 67% of thymoma patients are non-smokers, thymoma and smoking have a certain correlation which needs further exploration (5).
Thymic epithelial tumors are mainly classified into thymic neuroendocrine tumors, thymic carcinoma and thymoma. Thymoma is usually seen as a “relatively benign” tumor and has slow-proliferating biological behavior, with only the advanced stages capable of spreading locally in the thoracic cavity. Extrathoracic recurrence and metastases are rare and mainly occur with thymic neuroendocrine tumors or thymic carcinoma. According to the WHO histological classification criteria, thymoma is divided into five pathological subtypes (A, AB, B1, B2, B3). One hundred percent of patients with type A and AB, 83% patients with type B1 and B2, and 36% of patients with type B3 have disease-free survival rates of more than 10 years (6).
In this case, there was a difference between the type AB thymoma diagnosed and the biological behavior. Therefore, we need to continue to improve the WHO prognostic criteria. Another prognostic factor is the presence of capsular invasion. The recurrence rate of noninvasive thymoma is approximately between 0% and 7%, while invasive thymoma is approximately between 11% and 36%. The recurrence of thymoma mainly occurs in the mediastinum or the pleural cavity region. Extrathoracic metastases are extremely rare and main associated with type B (6). In the case of a metastasis, the site of distant metastasis is usually the lung and the liver, in turn.
In our case, PD of type AB thymoma was associated with the rapid progression of multiple metastases, and patients being insensitive to chemotherapy; thus, radiotherapy is critical for patient treatment (2,7). Compared with traditional radiotherapy, the beneficial characteristics of the TOMO radiotherapy are that the tumor dose is more conformable, the adjustment of the tumor dose intensity is more accurate, and the adjustment of the normal tissue dose around the tumor is finer. Once radiotherapy of multiple tumors lesion is achieved by spiral tomography of multiple subfields, the risk of repeated exposure of normal tissues can be reduced with complex planning. Additionally, because of its unique design, spiral illumination can achieve an ultra-long range intensity-modulated field (60 cm × 160 cm) and does not need to contend with the connection problem of adjacent fields.
However, the efficacy of TOMO on thymoma associated with multiple metastases has not yet been reported. In the present case, although the efficacy of TOMO radiotherapy was significant and the pain at the radiotherapy site was relieved, the degree of bone marrow suppression was beyond our expectations, leading to treatment termination. This suggests that accurate evaluation of tolerance before TOMO radiotherapy is very important. Here, we detailed the treatment process and outcomes for a patient with type AB thymoma associated with multiple metastases treated by TOMO for the first time. The obtained results encourage relevant clinical research to confirm whether TOMO could be the primary palliative therapeutic regimen for thymoma patients in phase IV.
Acknowledgments
I wish to thank all the authors for advice and help on the case report.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was reviewed and approved by Bengbu Medical University. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Hamaji M, Shah RM, Ali SO, et al. A Meta-Analysis of Postoperative Radiotherapy for Thymic Carcinoma. Ann Thorac Surg 2017;103:1668-75. [Crossref] [PubMed]
- Nagamata M, Okuma Y, Yamada Y, et al. Crucial role of treatment with palliative intent for a patient with advanced thymic carcinoma. Oncol Lett 2014;8:513-6. [Crossref] [PubMed]
- Girard N. Neuroendocrine tumors of the thymus: the oncologist point of view. J Thorac Dis 2017;9:S1491-500. [Crossref] [PubMed]
- Liu A, Gao X, Zhao L. Thymoma with acute gastric volvulus: a case report. BMC Cancer 2017;17:801. [Crossref] [PubMed]
- Passuello N, Pozza G, Blandamura S, et al. Thymoma metastatic to liver and pancreas: case report and review of the literature. J Int Med Res 2017;45:868-74. [Crossref] [PubMed]
- Kojima H, Isaka M, Nagata M, et al. Preoperative Proton Beam Therapy for Thymoma: A Case Report. Ann Thorac Cardiovasc Surg 2016;22:186-8. [Crossref] [PubMed]


