
Exosomes secreted by endothelial progenitor cells improve the bioactivity of pulmonary microvascular endothelial cells exposed to hyperoxia in vitro
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease with high morbidity and mortality in extremely preterm infants. Simplification of the pulmonary vasculature and alveolar is the pathological hallmark of BPD (1-4). Over 50 years since Dr. Northway first described this disorder, BPD has remained a critical clinical problem due to the lack of effective therapy (5).
A hyperoxic injury is one of the most commonly acknowledged etiologies of BPD, and the pulmonary capillary endothelium is the primary target cells in the initial burst in reactive oxygen species (6,7). Human infants dying from BPD, who had more days of oxygen supplementation, showed disrupted pulmonary vasculature with the decrease of angiogenesis-related factors, including VEGF, Flt-1, and TIE-2 (8). Hyperoxia exposure is a widely employed injurious stimulus to induce preclinical BPD model, resulting in inhibited distal microvasculature formation and an imbalance of pro- and anti-angiogenic factors in developing lungs (9-11).
Recent insights into the application of stem/progenitor cells to restore the lung development have provided us with the hope to find a new therapy for BPD. Endothelial progenitor cells (EPCs), precursors of endothelial cells, have been shown to promote the repair of damaged blood vessels in various disease models (12). In BPD rat model, exogenous EPCs transplanted through the peripheral vessels can migrate and engraft into the lungs of neonatal rats, improving vascularity and airspace development (13). Recent studies have reported that paracrine components of late EPCs also exert protection to BPD models by improving the growth of endothelial cells and alveolar cells, resulting in better lung structure and function (14,15). More recently, researchers have found that exosomes, the extracellular, membrane-bound nanoparticles that serve as an important paracrine factor, carry proteins, mRNA, and miRNA of EPCs and exchange their cargo with the endothelial cells of the target organ, serving as an additional mechanism for intercellular communication (16-18). Studies revealed that EPC-EXOs could transfer to endothelial cells, thus enhancing the function of endothelial cells to induce vascular formation and organ regeneration in various diseases (19-21).
However, the effect of EPC-EXOs on hyperoxic BPD model remains unclear; consequently, in this study, we established a hyperoxic cellular model of PMVECs via increasing the ambient oxygen concentration throughout the culture period, mimicking the process of BPD. Here, we attempt to investigate the potential role of EPC-EXOs in angiogenesis by mediating the activity of PMVECs under an external oxidative challenge, expecting to find an alternative therapy for BPD.
Methods
Cell culture and study design
The animal experiments were approved by the Animal Ethics Committee of the Children’s Hospital of Fudan University (No. 201687). The surgery was performed under anesthesia to minimize suffering.
PMVECs were purchased from Be Na Culture Collection Co., Ltd. (category No. BNCC338210; Beijing, China). PMVECs were cultured in DMEM culture media (Gibco, NY, USA) supplemented with 10% FBS (Gibco) and 100 U/mL Penicillin-Streptomycin Solution (Gibco). PMVECs were randomly divided into three groups: the control group, the hyperoxia group, and the EPC-EXOs group. The control group was exposed to normoxic gas (21% O2/5% CO2/74% N2), the hyperoxic group was exposed to high O2 gas (85% O2/5% CO2/10% N2), the EPC-EXO group was exposed to high O2 gas and EPC-EXOs (85% O2/5% CO2/10% N2 & 100 µg/mL exosomes). The plates of hyperoxia groups were placed in an airtight acryl box with moist tissues to maintain humidity, with O2 continually being infused into the box. The oxygen concentration in the box was continuously measured by monitor.
Isolation and identification of EPCs from rat bone marrow
The methods were performed as previously described with some modification (13). Briefly, mononuclear cells were isolated from the bone marrow of 10 days’ old SD rats using density gradient centrifugation with separation medium (Sigma, St. Louis, MO, USA). The isolated cells were placed into plates pre-coated with fibronectin (Sigma) and cultured in EGM-2MV (Lonza, Allendale, NJ, USA). After 48 h, non-adherent cells were discarded. Between the 7th and 10th days, cells were harvested for characterization of EPCs. The capacity to uptake Dil-ac-LDL and combined FITC-UEA-1 of the isolated cells were analyzed by fluorescence microscope. The expression of CD34 and VEGFR2 in EPC was assayed by immunocytochemistry with antibodies of anti-CD34 and VEGFR2 (Santa Cruz, CA, USA).
Isolation and identification of EPC-EXOs
When EPCs were 80% confluent, the cells were changed with the medium of EGM-2MV supplemented with 5% exosome-depleted FBS media supplement for an additional 48 h. The culture medium of EPCs was obtained and centrifuged at 2,000 ×g for 15 min to eliminate dead cells and cellular debris. The obtained medium underwent centrifugation at 10,000 ×g for 60 min, and the supernatant was then filtrated with 0.22 µm filter to remove cellular debris and large particles further. Exosomes were isolated using exosome precipitation solution (Exo-Quick; System Bioscience, MO, USA) following the manufacturer’s instructions. The exosome pellet was resuspended with PBS and stored at −80 °C for subsequent study.
The ultrastructure and size distribution of exosomes were analyzed by transmission electron microscopy and NanoSight (Malvern, UK) respectively. The characteristic exosomal surface marker proteins of CD63 and TSG101 (Abcam, Cambridge, UK) were determined by western blotting.
Internalization assay of EPC-EXOs
Purified exosomes were labeled with a PKH67(green) kit (Sigma-Aldrich) according to the manufacturer’s instructions. The labeled exosomes were suspended with PBS and incubated with PMVECs. After cells were fixed, they were visualized under a fluorescence microscope.
Proliferation assay
To investigate the effect of EPC-EXOs on cell proliferation, PMVECs were seeded on 6 cm culture plates with a density of 5×105 cells. The plates were exposed to the indicated oxygen concentration. Pictures were taken every 24 h using a phase-contrast microscope. Cells numbers of three randomly chosen fields of every plate were counted using Image J software at the end of timepoint.
Tube formation assay
To assay in vitro angiogenesis, PMVECs (10,000 cells/well) were seeded onto 96-well plates coated by Matrigel and incubated in different conditions. The culture medium was changed every 24 h and cells were incubated for 72 h. The capillary-network formation was monitored using an inverted microscope. The number of the nodes in three randomly chosen fields of every plate was examined.
Migration assay
PMVEC migration was analyzed using 8.0 µm Transwell inserts (Corning, 3422). In detail, 900 µL of DMEM medium was added to the lower chamber, while 1×105 PMVECs in 200 µL of DMEM medium deprived of FBS were seeded on the upper chamber of Transwell inserts. Seventy-two hours after incubation in different conditions, suspension cells were removed, and cells on each insert were removed with cotton swabs. The membranes were fixed with 4% paraformaldehyde for 15 min at room temperature. The migrated cells were stained with Crystal Violet Staining Solution (Solarb, China) for 15 min, then rinsed with PBS three times, and counted under the microscope in five microscopic random fields.
qRT-PCR analysis
PMVECs (5×105 cells per well) were seeded in 6 cm plates and cultured in DMEM media supplemented with exosomes in indicated oxygen concentration for 48 h. Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was synthesized from 1 µg of total RNA by using the PrimeScriptTM RT reagent Kit with gDNA (Takara Biotechnology, Japan). Then, qRT-PCR was performed with a Roche LightCycler 480 System with SYBR Premix ExTaqTM II (Takara Biotechnology, Japan). β-ACTIN was used as a housekeeping gene for internal normalization. Primers used in the amplification reaction were synthesized as the following: VEGF: forward, 5'-GCAGATGTGACAAGCCAAGG-3', and reverse, 5'-GATGGTGGTGTGGTGGTGAC-3'; VEGFR2: forward, 5'-ACAGCATCACCAGCAGTCAG, and reverse, 5'-GATGCTCCAAGGTCAGGAAG-3'; eNOS: forward, 5'-ACTATGGCAACCAGCGTCCT, and reverse, 5'-CTCGTGGTAGCGTTGCTGAT-3'; β-ACTIN: forward, 5'-TGTCACCAACTGGGACGATA, and reverse, 5'-GGGGTGTTGAAGGTCTCAAA-3'. After validation of amplification efficiencies of target genes and the internal control gene, quantification of target gene expression was calculated using a 2−ΔΔCT method.
Western blotting analysis
After different treatments, proteins from PMVECs were obtained with lysis buffer supplemented with protease inhibitor cocktail (Thermo Scientific, FL, USA). Cell debris was removed by centrifugation at 12,000 ×g, for 30 min at 4 °C. Then, the cell lysates were separated by SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked by incubating with 10% dry milk for 2 h and then incubated with primary antibodies against VEGF, VEGFR2, and eNOS in a concentration of 1:2,000 at 4 °C overnight. All the antibodies were purchased from Abcam (Cambridge, UK). GAPDH (1:4,000; Sigma-Aldrich, St. Louis, MO, USA) was used to normalize protein loading. After being washed thoroughly, membranes were incubated with horseradish peroxidase-(HRP) conjugated IgG (1:5,000; Cell Signaling Technology, MA, USA) for 2 h at room temperature. Blots were then developed with enhanced chemiluminescence developing solutions and quantified by Image J software.
Statistical analysis
All experiments were performed in triplicate. The data were shown as mean ± standard deviation (SD). Differences were analyzed using one-way analysis of variance (ANOVA) with SPSS 22.0, followed by post hoc. Bonferroni testing for between-group differences. P values <0.05 were considered statistically significant.
Results
Identification of rat bone marrow-derived EPCs
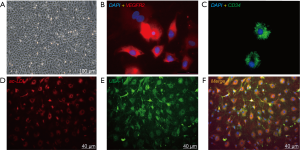
Bone marrow-derived mononuclear cells were isolated by density gradient centrifugation and cultured in endothelial-specific conditions. The cells developed the typical endothelial-like cobblestone morphology at day 7 (Figure 1A). Immunostaining results showed that EPC colonies expressed endothelial lineage markers of VEGFR-2 and CD34 (Figure 1B,C). They demonstrated the ability to uptake Dil-ac-LDL and bind FITC-UEA-1 (Figure 1D,E,F).
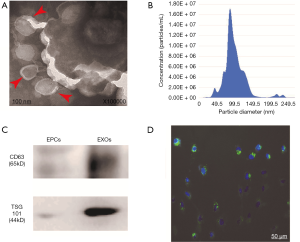
Identification and characterization of exosomes from EPCs
Exosomes isolated from EPCs demonstrated biconcave or cup-shaped morphology under transmission electron microscopy (Figure 2A). Nanosight analysis showed that the size of EPC-EXOs was approximately 50–150 nm (Figure 2B). Western blotting exhibited that exosomal marker proteins CD63 (exosomal surface marker protein) and TSG101 (exosomal luminal protein) were present in these exosomes (Figure 2C). To further investigate whether the EPC-EXOs could be transferred into endothelial cells, exosomes were labeled with PKH67 and incubated with PMVECs in vitro. The uptake was confirmed by fluorescence microscopy. After 12 h, over 95% of PMVECs were PKH67 positive (Figure 2D).
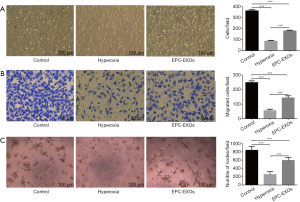
EPC-EXOs improved the angiogenic activity of PMVECs in increased oxygen risk
Effects of EPC-EXOs on PMVECs proliferation
There was a significant difference in the proliferation capacity of PMVECs among the three groups (Figure 3A). Compared to the control group, hyperoxia significantly inhibited PMVEC proliferation, as indicated by cell density and manual cell counting (362±9.20 vs. 87.0±7.17, cells/field, P<0.001). EPC-EXOs improved PMVEC proliferation compared to the hyperoxia group (180±5.62 vs. 87±7.17, cells/field, P<0.001).
Effects of EPC-EXOs on PMVEC migration
Transwell assay was used to determine the migration of PMVECs after treatment by exosomes. There was a significant difference among the three groups (Figure 3B). In comparison with normal group, it was shown that hyperoxia exposure weakened the motility of PMVECs (248±11.6 vs. 55.3±10.3, cells/field, P<0.001), as indicated by the number of cells in the Transwell assay. EPC-EXOs could promote the migration of PMVECs compared to the H group (144±13.7 vs. 55.3±10.3, cells/field, P<0.001).
Effects of EPC-EXOs on PMVEC tube formation
To further explore the pro-angiogenic role of EPC-EXOs on PMVECs in vitro, a tube formation assay was performed. There was a significant difference among the three groups (Figure 3C). Results showed tube formation of PMVECs was significantly impaired in the hyperoxia group as determined by the number of nodes, in comparison to the normal group (840±86.5 vs. 253±65.0, nodes/field, P<0.001). The PMVECs could not form any tubes but cell clusters. EPC-EXOs improved the vascular formation ability of PMVECs (595±65.8 vs. 253±65.0, nodes/field, P<0.001).
EPC-EXOs improved angiogenesis-related molecules expression of PMVECs in increased oxygen risk
To test whether angiogenesis-related molecules expression was altered by internalization of EPC-EXOs, the mRNA and protein levels of eNOS, VEGF, and VEGFR-2 were evaluated by qRT-PCR and western blot analysis. There was a significant difference among the three groups. As shown in Figure 4, hyperoxic stress down-regulated the mRNA and protein levels of eNOS, VEGF and VEGFR-2. After stimulated with EPC-EXOs, the levels of eNOS, VEGF and VEGFR-2 were up-regulated (Figure 4A,B).
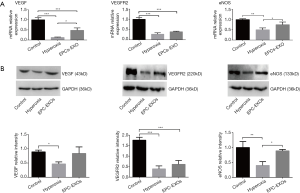
Compared to the control group, the VEGF mRNA expression was significantly decreased in the hyperoxia groups (1.00±0.11 vs. 0.13±0.02, P<0.001), and the VEGF mRNA in the EPC-EXO treatment group was higher than that of the hyperoxia group (0.48±0.10 vs. 0.13±0.02, P<0.05). Compared to the control group, the VEGF protein expression was significantly decreased in the hyperoxia group (0.89±0.06 vs. 0.47±0.07, P<0.05), and the level of VEGF protein in EPC-EXOs treatment group was higher than that of the hyperoxia group, though the difference was not statistically significant.
Compared to the control group, VEGFR2 mRNA and protein expressions were significantly decreased in hyperoxia (1.00±0.09 vs. 0.28±0.07, 1.77±0.14 vs. 0.40±0.14, P<0.001, respectively) and the EPC-EXOs] groups (1.00±0.09 vs. 0.39±0.03, 1.77±0.14 vs. 0.61±0.18, P<0.001, respectively). The levels of VEGFR2 mRNA and protein in the EPC-EXO group were higher than those of the hyperoxia group, though the difference was not statistically significant. Compared to the control group, eNOS mRNA and protein expressions were decreased in the hyperoxia group (1.00±0.04 vs. 0.50±0.09, 1.00±0.20 vs. 0.40±0.13, P<0.01, respectively) and the EPC-EXO group (1.00±0.04 vs. 0.75±0.13, 1.00±0.20 vs. 0.89±0.05, P>0.05, respectively). The levels of eNOS mRNA and protein in the EPC-EXOs group were higher than those of the hyperoxia group (0.75±0.13 vs. 0.50±0.09, 0.89±0.05 vs. 0.40±0.13, P<0.05, respectively).
Discussion
Our results revealed that hyperoxia exposure impaired the capacity to proliferate, migrate, and form the vessel-like structures of PMVECs, and downregulated the expression of angiogenesis-related molecules, including VEGF, VEGFR2, and eNOS. We also found that EPC-EXOs preserved the proliferation, migration, and tube formation of PMVECs under oxidative stress and increased the expression of VEGF, VEGFR2, and eNOS, which were important angiogenesis-related molecules during lung development.
Oxidative stress may induce lung endothelial cells injury by vastly complex cellular and molecular pathways. It has been reported that hyperoxia exposure directly causes oxidative injury to endothelial cells, damaging the organelles (22), inhibiting cell cycles (23-25), and interfering with the expression of growth factors associated with vascular development (10,26), which are key events of angiogenesis. We found that hyperoxia exposure disturbed the bioactivities of PMVECs, particularly in that they could not form any tubes in vitro but only cell clusters, which might reflect the malformation or scarceness of the microvascular structures in BPD. Important growth factors associated with angiogenesis, including VEGF/VEGFR2 and eNOS were decreased in PMVECs exposed to hyperoxia, which was consistent with the above studies. Many studies revealed that lung vascular endothelium had a particular susceptibility to hyperoxia, and capillary endothelial damage is more extensive and typically occurs earlier than damage to the alveolar epithelium in preclinical hyperoxic lung injury models (27,28).
Taken together, PMVECs dysfunction by hyperoxia exposure may be associated with pulmonary blood vessel loss and vascular remodeling in BPD.
EPC and/or its paracrine factors-based therapies have emerged as a promising treatment option for various diseases and an area of intense research interest. Recently, the effects of extracellular vesicles derived from EPCs have been explored in cardiovascular and renal diseases (29,30). The exosomes are important extracellular vesicles. In this study, we found EPC-EXOs exhibited a cup or biconcave morphology, with the size ranging from 30 to 150 nm and positive for the characteristic exosomal surface marker proteins of CD63 and TSG101, which were morphologically similar in size and shape to exosomes described in previous reports, carrying known exosomal protein markers. Immunofluorescence assays confirmed that PKH67-labeled exosomes could be taken up into the PMVECs. Taken together, these observations showed that EPC-EXOs could be successfully isolated and efficiently transferred to PMVECs.
We found that EPC-EXO treatment induced the angiogenic activity of PMVECs cultured under external oxidative challenge in vitro. We observed that EPC-EXOs better preserved PMVEC proliferation and migration compared to the hyperoxia group. The PMVECs could not form any tubes but only cell clusters in the hyperoxia group. After EPC-EXO treatment, the vascular formation ability of PMVECs improved. Overall, EPC-EXOs showed protective effects in angiogenesis-related processes including proliferation, migration, and tube formation on PMVECs exposed to hyperoxia. Other studies also showed that EPC-EXO treatment could improve survival, proliferation, and tube formation capability of endothelial cells of various types under different stresses (20,21,31).
In this study, EPC-EXO treatment increased the expression of angiogenic factors of PMVECs, including VEGF/VEGFR2 and eNOS, which were downregulated in the hyperoxic group. VEGF/VEGFR2s are important signals for angiogenesis during lung development, and eNOS/NO pathway is associated with BPD (32). VEGF phosphorylates VEGFR-2 and its downstream signaling activates eNOS. Blocking this pathway augmented smoking-induced oxidative stress and inflammatory responses leading to endothelial dysfunction, indicating its key role in the regulation of endothelial cell migration, proliferation, and survival under oxidant stress (33,34). This suggests that EPC-EXOs may contribute therapeutic benefits to PMVECs by modulating the imbalance of pro-angiogenic factors induced by hyperoxia exposure. Li also reported various critical pro-angiogenic genes of human microvascular endothelial cells cultured in normal condition, including eNOS, interleukin-8, angiopoietin-1, and E-selectin, were significantly up-regulated after stimulation with EPC-EXOs (31). A recent report revealed that additional pathways might play a role in EPC-EXO-mediated angiogenesis, showing that EPC-EXOs suppressed lung vascular leakage in septic mice and attenuated the increases in plasma levels of LPS-induced high mobility group box 1 and vascular cell adhesion molecule 1 in human microvascular endothelial cells (35). Interestingly, exosomes from mesenchymal stem cells, another alternative stem cell for treating BPD, has been shown to promote microvascular and airspace development in hyperoxic BPD mouse model by blunting pro-inflammatory signaling and immune responses in the hyperoxic lung via modulation of lung macrophage phenotype (36). Thus, EPC-EXOs appear to have promise in facilitating vascular repair in BPD model induced by hyperoxia.
There are limitations to this study. Firstly, the exact cargo of EPC-EXOs is still incompletely understood, and the exact molecular mechanisms of their therapeutic action on PMVECs need to be elucidated further. Secondly, the current research is a cellular study in vitro; the protective effect of EPC-EXOs on BPD animal models should be verified further.
In summary, our study showed that EPC-EXOs are active components of EPC paracrine secretion, which promotes angiogenesis by improving endothelial cell functions like proliferation, tube formation, and cell migration, when under oxidative challenge. These effects are associated with the expression of VEGF, VEGFR2, and eNOS. The exact mechanism and study in vivo should be considered further.
Acknowledgments
Funding: This work is supported by grants from the National Natural Science Foundation of China (Grant No.: 81471481 and 81601332).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The animal experiments were approved by the Animal Ethics Committee of the Children’s Hospital of Fudan University (No. 201687).
References
- Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. [Crossref] [PubMed]
- Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175:978-85. [Crossref] [PubMed]
- Guaman MC, Gien J, Baker C, et al. Point Prevalence, Clinical Characteristics, and Treatment Variation for Infants with Severe Bronchopulmonary Dysplasia. Am J Perinatol 2015;32:960-7. [Crossref] [PubMed]
- Kong X, Xu F, Wu R, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatrics 2016;16:174. [Crossref] [PubMed]
- Abman SH, Bancalari E, Jobe A. The Evolution of Bronchopulmonary Dysplasia after 50 Years. Am J Respir Crit Care Med 2017;195:421-4. [Crossref] [PubMed]
- Buczynski BW, Maduekwe ET, O, Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Seminars in Perinatology 2013;37:69-78. [Crossref] [PubMed]
- Madurga A, Mižíková I, Ruiz-Camp J, et al. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2013;305:L893-905. [Crossref] [PubMed]
- Bhatt AJ, Pryhuber GS, Huyck H, et al. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971-80. [Crossref] [PubMed]
- Yee M, White RJ, Awad HA, et al. Neonatal Hyperoxia Causes Pulmonary Vascular Disease and Shortens Life Span in Aging Mice. Am J Pathol 2011;178:2601-10. [Crossref] [PubMed]
- Wagenaar GT, ter Horst SA, van Gastelen MA, et al. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 2004;36:782-801. [Crossref] [PubMed]
- Bland RD, Mokres LM, Ertsey R, et al. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol 2007;293:1099-110. [Crossref] [PubMed]
- Guo Y, Tang W, Liu Q, et al. Endothelial progenitor cell therapy: From bench to bedside. Int J Cardiol 2016;208:164-5. [Crossref] [PubMed]
- Lu A, Sun B, Qian L. Combined iNO and endothelial progenitor cells improve lung alveolar and vascular structure in neonatal rats exposed to prolonged hyperoxia. Pediatr Res 2015;77:784-92. [Crossref] [PubMed]
- Di Santo S, Yang Z, Wyler VB, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One 2009;4:e5643. [Crossref] [PubMed]
- Alphonse RS, Vadivel A, Fung M, et al. Existence, Functional Impairment, and Lung Repair Potential of Endothelial Colony-Forming Cells in Oxygen-Induced Arrested Alveolar Growth. Circulation 2014;129:2144-57. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-59. [Crossref] [PubMed]
- Ferguson SW, Nguyen J. Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J Control Release 2016;228:179-90. [Crossref] [PubMed]
- He C, Zheng S, Luo Y, et al. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018;8:237-55. [Crossref] [PubMed]
- Wang J, Chen S, Ma X, et al. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev 2013;2013:572729. [Crossref] [PubMed]
- Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications 2016;30:986-92. [Crossref] [PubMed]
- Zhang J, Chen C, Hu B, et al. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int J Biol Sci 2016;12:1472-87. [Crossref] [PubMed]
- Nakanishi H, Morikawa S, Kitahara S, et al. Morphological characterization of pulmonary microvascular disease in bronchopulmonary dysplasia caused by hyperoxia in newborn mice. Med Mol Morphol 2018;51:166-75. [Crossref] [PubMed]
- Wu J, Hafner C, Schramel JP, et al. Cyclic and constant hyperoxia cause inflammation, apoptosis and cell death in human umbilical vein endothelial cells. Acta Anaesthesiol Scand 2016;60:492-501. [Crossref] [PubMed]
- Hafner C, Wu J, Soto-Gonzalez L, et al. Moderate hyperoxia induces inflammation, apoptosis and necrosis in human umbilical vein endothelial cells: An in-vitro study. Eur J Anaesthesiol 2017;34:141-9. [PubMed]
- Bik-Multanowski M, Revhaug C, Grabowska A, et al. Hyperoxia induces epigenetic changes in newborn mice lungs. Free Radic Biol Med 2018;121:51-6. [Crossref] [PubMed]
- Willam C, Schindler R, Frei U, et al. Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am J Physiol 1999;276:H2044-52. [PubMed]
- Fracica PJ, Knapp MJ, Piantadosi CA, et al. Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Physiol 1991;71:2352-62. [Crossref] [PubMed]
- Barazzone C, Horowitz S, Donati YR, et al. Oxygen Toxicity in Mouse Lung: Pathways to Cell Death. Am J Respir Cell Mol Biol 1998;19:573-81. [Crossref] [PubMed]
- Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012;82:412-27. [Crossref] [PubMed]
- Gu S, Zhang W, Chen J, et al. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One 2014;9:e85396. [Crossref] [PubMed]
- Li X, Chen C, Wei L, et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016;18:253-62. [Crossref] [PubMed]
- Balasubramaniam V, Maxey AM, Morgan DB, et al. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L119-27. [Crossref] [PubMed]
- Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signaling–in control of vascular function. Nat Rev Mol Cell Biol 2006;7:359-71. [Crossref] [PubMed]
- Edirisinghe I, Yang SR, Yao H, et al. VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction. FASEB J 2008;22:2297-310. [Crossref] [PubMed]
- Zhou Y, Li P, Goodwin AJ, et al. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol Ther 2018;26:1375-84. [Crossref] [PubMed]
- Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med 2018;197:104-16. [Crossref] [PubMed]


