
Prognostic value of lymphovascular invasion in patients with esophageal squamous cell carcinoma
Introduction
Esophageal cancer is a highly aggressive malignancy. This cancer is the sixth leading cause of cancer-related death globally and is associated with 400,000 deaths each year (1,2). Despite the recent diagnostic and therapeutic advances in esophageal cancer treatment, the majority of esophageal cancer patients die within 2 years of diagnosis, and the 5-year survival rates appear to plateau at approximately 19–36% (3-6). A valid staging system for esophageal cancer is the premise for determining suitable treatment options and the long-term survival recommendations (7,8). As the three most important predictors, T (primary tumor), N (lymph node), and M (metastasis) are the cornerstones of the current stage classification in esophageal squamous cell carcinoma (ESCC) (2). In addition, several other characteristics have also been reported as independent predictors, including tumor location, histology, differentiation, and circumferential resection margin (9-12). Whether lymphovascular invasion (LVI) in ESCC should be recognized as an independent prognostic factor for survival is a controversial issue in clinical research (13). In this study, we aimed to determine the value of LVI status in predicting the overall survival (OS) of ESCC patients.
Methods
Patients
A total of 209 consecutive esophageal cancer patients were reviewed retrospectively. All of the patients had undergone curative esophagectomy at West China Hospital from October 2010 to July 2011. A total of 57 patients were excluded due to following reasons: (I) adenocarcinoma (n=25); (II) in-hospital mortality within 30 days post-surgery (n=8); (III) M1 stage confirmed during operation (n=2); (IV) positive esophageal proximal resection margin (n=12); (V) incomplete pathological data (n=7); and (VI) preoperative chemoradiotherapy (n=3). This study was approved by the Ethics Committee of West China Hospital.
All patients were treated with radical resection. Left thoracotomy (Sweet) and right thoracoabdominal (Ivor-Lewis) were performed for tumors located in the lower 2/3 of the esophagus, as long as there was no clinical indication of superior mediastinum or neck lymph node metastasis (LNM). Triple incision (McKeown) minimally invasive esophagectomy was used for middle and upper thoracic esophageal cancers or when clinical indications/suspicions existed regarding superior mediastinum or neck LNM. Intrathoracic anastomosis was performed in Ivor-Lewis and Sweet approach, while cervical anastomosis was conducted in McKeown approach. The hand-sewn anastomosis was constructed with interrupted single- or double-layer suture. And the mechanical anastomosis was performed by circular or liner stapler.
Pathologic examination
All resected specimens were examined macroscopically prior to fixation in 4% formaldehyde. In order to ensure the accurate staging of all patients, the features of tumor, the lymph node involvement, and LVI were reexamined. The 8th edition of the American Joint Committee on Cancer TNM staging system was used to determine the pathologic stages. LVI was defined as the presence of neoplastic cells within an arterial, venous, or lymphatic lumen during routine histologic evaluation with H&E. In the final synoptic report, purely arterial, venous, or lymphatic invasion were not distinguished during the pathological review, so subgroup analyses based on these subtypes are not available.
Follow-up
In the first 2 years, all patients were seen for follow-up visits once every three months and semiannually thereafter. History taking, physical examination, chest/abdominal CT scans, and contrast esophagography were routinely performed as part of the follow-up protocol. If clinically indicated, the patients were referred for abdominal ultrasound, radionuclide bone scans, PET-CT scans, and upper GI endoscopy. Death within the first 30 days following surgery and in-hospital deaths were defined as operative mortality.
Statistical analysis
Chi-square or Fisher’s exact test was used for comparisons of categorical data between the two groups. Comparison of continuous variables was made by two-tailed t test or Wilcoxon rank-sum test. OS curves were calculated using the Kaplan-Meier method, and the log-rank test was used to compare the differences between survival curves. Univariate and multivariate analyses were performed with the Cox proportional hazards regression model using enter stepwise regression. Statistically significant variables (P<0.05) were entered into the multivariate analysis.
Results
Patient characteristics
The demographic information of these 152 patients and the correlation between LVI and clinicopathological variables are listed in Table 1. There were 131 men and 21 women with a median age of 59 years old (range, 36–78 years old). Nearly half of the tumors were found in the middle thoracic esophagus. LVI was discovered in 49 patients (32.2%). There was a significant association between LVI within the N category and tumor differentiation. No significant differences were found in other clinicopathological features between the patients with or without LVI (Table 1).
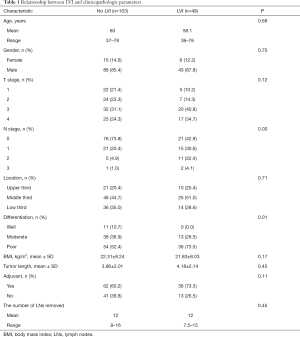
Full table
Predictors of OS
The median follow-up time for all patients was 44.76 months. The 1-, 3-, and 5-years OS rates were 82.2%, 58.6%, and 41.3%, respectively. Patients without LVI had a significantly better 5-year OS rate than those with positive LVI (52.9% vs. 28.8%; P=0.000) (Figure 1). Age, T status, N status (P=0.000), differentiation, and LVI manifested as significant prognostic factors for OS through univariate analyses (Table 2). Age (P=0.018), T status (P=0.008), N status (P=0.000), and LVI (P=0.005) were confirmed, by multivariate analysis, as independent prognostic factors for determine OS (Table 2).
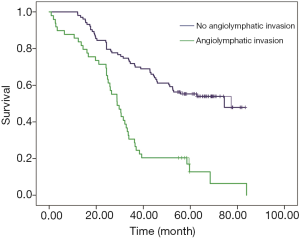
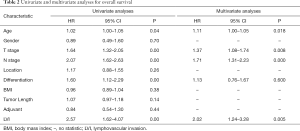
Full table
Controlling for T and N status
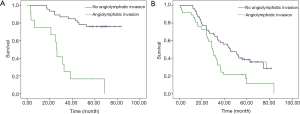
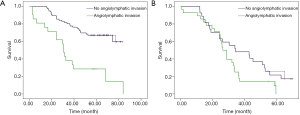
To evaluate the effect of esophageal LVI on the depth of tumor invasion (T1/2 and T3/4) and lymph node status (node negative and node positive), patients were stratified into different groups. The results showed that LVI had a significantly negative influence on OS in both the T1/2 group and the T3/4 group (Figure 2A, P=0.000; Figure 2B, P=0.008). In the node-negative subgroup, patients without LVI had an improved OS relative to patients with LVI (P=0.000, Figure 3A). In the node-positive subgroup, no significant difference was observed in OS according to LVI status (P=0.074, Figure 3B).
Discussion
In the current study, we assessed a retrospective collection of data on patients with ESCC in order to determine the prognostic value of LVI. The positive rate of LVI in this cohort was 32.2%, which was comparable with the rate reported in the literature (14-18). Patients with LVI had a significantly decreased OS compared with those without LVI detection. In further, LVI involvement was remarkably associated with the well-established features of biologically aggressive ESCC, such as poor tumor differentiation and metastases to lymph nodes. In addition, our multivariate Cox analysis demonstrated that LVI was strongly associated with OS in ESCC patients, a prognostic factor independent of certain well-established clinical factors, including tumor differentiation, pT status, pN status and age.
Our findings of LVI were in accordance with previously published articles. Sugimachi et al. reported a study of 128 patients to determine the prognostic significance of various clinical and histopathological factors for ESCC (19). These researchers reported that the presence of LVI significantly decreased the 5-year survival rate. The adverse effect of LVI on survival was observed in 3 studies: Tanaka et al. (20), including 105 patients; Zhu et al. (21), including 207 patients; and Ide et al. (22), including 403 patients. Through multivariate analyses, however, the trials by Tanaka et al. (20) and Zhu et al. (21) did not demonstrate that LVI was an independent prognostic factor for survival. With a focus on evaluating LVI in superficial ESCC, Mitobe et al. found that LVI was a reliable indicator of the risk of LNM (23). In advanced esophageal cancer, Schiefer et al. demonstrated that LVI in lymph node with tumor cells metastasis still had a negative impact on survival in esophageal cancer patients (24).
In our study, we grouped patients by well-established independent prognostic factors for esophageal cancers (7). LVI remained a prognostic factor after controlling for depth of invasion, and its predictive value regarding OS was significant for both T1/2 and T3/4 lesions (Figure 2). Additionally, we stratified patients by lymph node status, which is the other strongest prognostic factor in patients with esophageal cancer (7,25,26). Our study showed that LVI was associated with a decreased OS in node-negative patients only, and no significant difference was found in node-positive patients with LVI. Theoretically, the LVI is a process of LNM (27). In other words, the presence of LVI is a necessary condition but not a sufficient condition for LNM. Hence, it is reasonable to frame the hypothesis that LVI may be a prognostic marker essentially equivalent to LNM. This hypothesis may explain the results that LVI failed to be an independent prognostic factor in patients with node-positive esophageal cancer (18).
This study had several limitations, such as its small sample size and retrospective nature. Currently, neoadjuvant chemoradiotherapy (nCRT) followed by surgery has been repeatedly advocated as the standard treatment for locally advanced esophageal cancer, especially for ESCC, which seems to be more sensitive to CRT (28-30). This induction strategy, however, had not been widely adopted until 2012 (31,32), and there was insufficient data in our cohort [2010–2011] to demonstrate the significance and feasibility of LVI status after nCRT.
Conclusions
Our study demonstrated that LVI was significantly associated with poorer OS in ESCC patients. LVI may provide additional information when determining postoperative treatment strategy.
Acknowledgments
The authors gratefully acknowledge Du He for his help in the assessment of pathological features.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of West China Hospital (No. 201649).
References
- Lightdale CJ. Esophageal cancer. American College of Gastroenterology. Am J Gastroenterol 1999;94:20-9. [Crossref] [PubMed]
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Thomas P, Doddoli C, Lienne P, et al. Changing patterns and surgical results in adenocarcinoma of the oesophagus. Br J Surg 1997;84:119-25. [Crossref] [PubMed]
- Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249-60. [Crossref] [PubMed]
- Ohashi S, Miyamoto S, Kikuchi O, et al. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology 2015;149:1700-15. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002;95:1434-43. [Crossref] [PubMed]
- Rice TW, Adelstein DJ. Precise clinical staging allows treatment modification of patients with esophageal carcinoma. Oncology (Williston Park) 1997;11:58-62. [PubMed]
- Shi H, Zhang K, Niu ZX, et al. Does tumour location influence postoperative long-term survival in patients with oesophageal squamous cell carcinoma? Eur J Cardiothorac Surg 2015;48:266-72. [Crossref] [PubMed]
- Hulshoff JB, Faiz Z, Karrenbeld A, et al. Prognostic Value of the Circumferential Resection Margin in Esophageal Cancer Patients After Neoadjuvant Chemoradiotherapy. Ann Surg Oncol 2015;22 Suppl 3:S1301-9. [Crossref] [PubMed]
- Hollis AC, Quinn LM, Hodson J, et al. Prognostic significance of tumor length in patients receiving esophagectomy for esophageal cancer. J Surg Oncol 2017;116:1114-22. [Crossref] [PubMed]
- Wang YC, Deng HY, Wang WP, et al. Positive esophageal proximal resection margin: an important prognostic factor for esophageal cancer that warrants adjuvant therapy. J Thorac Dis 2016;8:2512-18. [Crossref] [PubMed]
- Wang S, Chen X, Fan J, et al. Prognostic Significance of Lymphovascular Invasion for Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2016;23:4101-9. [Crossref] [PubMed]
- Jia R, Luan Q, Wang J, et al. Analysis of Predictors for Lymph Node Metastasis in Patients with Superficial Esophageal Carcinoma. Gastroenterol Res Pract 2016;2016:3797615. [Crossref] [PubMed]
- Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008;112:1020-7. [Crossref] [PubMed]
- Reid TD, Chan DS, Roberts SA, et al. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer 2012;107:1925-31. [Crossref] [PubMed]
- Wu J, Chen QX, Shen DJ, et al. A prediction model for lymph node metastasis in T1 esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2018;155:1902-08. [Crossref] [PubMed]
- Huang Q, Luo K, Chen C, et al. Identification and Validation of Lymphovascular Invasion as a Prognostic and Staging Factor in Node-Negative Esophageal Squamous Cell Carcinoma. J Thorac Oncol 2016;11:583-92. [Crossref] [PubMed]
- Sugimachi K, Matsuura H, Kai H, et al. Prognostic factors of esophageal carcinoma: univariate and multivariate analyses. J Surg Oncol 1986;31:108-12. [Crossref] [PubMed]
- Tanaka T, Matono S, Nagano T, et al. Esophagectomy with extended lymphadenectomy for submucosal esophageal cancer: long-term outcomes and prognostic factors. Ann Surg Oncol 2012;19:750-6. [Crossref] [PubMed]
- Zhu Z, Yu W, Li H, et al. Nodal skip metastasis is not a predictor of survival in thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 2013;20:3052-8. [Crossref] [PubMed]
- Ide H, Nakamura T, Hayashi K, et al. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg 1994;18:321-30. [Crossref] [PubMed]
- Mitobe J, Ikegami M, Urashima M, et al. Clinicopathological investigation of lymph node metastasis predictors in superficial esophageal squamous cell carcinoma with a focus on evaluation of lympho-vascular invasion. Scand J Gastroenterol 2013;48:1173-82. [Crossref] [PubMed]
- Schiefer AI, Schoppmann SF, Birner P. Lymphovascular invasion of tumor cells in lymph node metastases has a negative impact on survival in esophageal cancer. Surgery 2016;160:331-40. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Nathanson SD, Kwon D, Kapke A, et al. The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol 2009;16:3396-405. [Crossref] [PubMed]
- Cox SJ, O'Cathail SM, Coles B, et al. Update on Neoadjuvant Regimens for Patients with Operable Oesophageal/Gastrooesophageal Junction Adenocarcinomas and Squamous Cell Carcinomas. Curr Oncol Rep 2017;19:7. [Crossref] [PubMed]
- Tomasello G, Petrelli F, Ghidini M, et al. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol 2017;43:1607-16. [Crossref] [PubMed]
- Koëter M, Parry K, Verhoeven RH, et al. Perioperative Treatment, Not Surgical Approach, Influences Overall Survival in Patients with Gastroesophageal Junction Tumors: A Nationwide, Population-Based Study in The Netherlands. Ann Surg Oncol 2016;23:1632-8. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Lanuti M. Early-stage (cT2N0) esophageal cancer: Should induction therapy be a standard? J Thorac Cardiovasc Surg 2018;155:2231-2. [Crossref] [PubMed]


