Management of air leaks post-surgical lung resection
Introduction
Air leaks also known as alveolar-pleural fistulas (APL) remain a challenge for the thoracic surgeon and one of the most common if not the most common complications post elective lung resection. Despite advances in surgical technology and techniques air leaks are the leading cause of increased morbidity in patients that can result in prolonged hospital time, increased pain from prolonged chest tubes, increased overall costs, empyema, pneumonia and overall patient dissatisfaction (1).
Numerous causes of airleaks have been identified including trauma secondary to lung manipulation, fissure dissection, barotrauma, or a result of underlying lung disease. Air leak rates have varied in the literature but most large series report a rate between 20–33% after elective pulmonary resection (2-6). Majority of air leaks will seal on their own, within 5 days of surgery and are management with tube thoracostomy and observation. Many authors use the term persistent air leak (PAL) which is often defined as airleaks that last longer than 5–7 days post-operative, however no true consensus exists on what is actually the defined amount of time to classify a PAL (6-8). Air leaks can be graded to the Cerfolio classification (Table 1) and the newer grading system developed by Sang et al. (Table 2). Multiple risk factors for PAL have been described which include low forced expiratory volume in 1 second (FEV1), preexisting lung pathology, pleural adhesions requiring adenolysis, steroids, previous chemotherapy, previous radiation, immunocompromised patients, and diabetes (1).
Full table

Full table
This is a review article that will review several techniques in a stepwise approach that can help seal persistent airleaks and ultimately allow the patient to leave the hospital and continue on with recovery after surgery. Initial strategies include conservative therapy with observation and taking the chest tube off continuous suction and placing the chest tube to water seal. Fibrin sealants (9-15) and pleurodesis with doxycycline (16-18) can be put through the chest tube at the bedside or in the OR and are a good option for noninvasive modalities for PAL. Endobronchial valves (EBVs) are a new endoscopic technique which is being used to help deal air leaks. Finally return to OR for operative intervention is the last strategy we will review for the management of PAL. Lastly patients with PAL can be sent home with a mini 500 pleuravac as an alternative option assuming the lung has re-expanded and there is adequate teaching and ability to care for the tube and device.
Management strategies
Conservative management with water seal and observation
Traditionally the use of suction −20 cmH2O to chest tubes has been used by thoracic surgeons following lung resections. However, many authors have questioned the evidence behind this suction, and many feels that suction may even lead to prolonged air leaks, while others feel that all patients should remain on suction postoperatively (19,20). Many believe that water seal promotes healing of the airleak by decreasing the amount of air escaping out the lung, while others believe suction removes the air in the pleural space allowing for apposition between the parietal and visceral pleura (1).
The use of suction vs. water seal for post-operative air leaks was first investigated by Cerfolio et al. when he randomized 33 patients who had air leaks on post-operative day 2 after elective lung resection. He found that group randomized to water seal, 67% had resolved their airleak on POD 3, compared to 7% of patients randomized to suction at −20 cmH2O (21). Since Cerfolio’s first randomized control study, other studies have shown similar results such as the one published by Marshall et al. He randomized 68 patients right from the OR and he found the airleak sealing was significantly shortly in the water seal group (1.5) days, compared to the suction group of 3.3 days (22).
The use of water seal immediately following pulmonary resection has clearly been shown to reduce total air leak time, however one must consider options if a pneumothorax is persistent on water seal or a persistent air leak is present. If a pneumothorax is present suction should be started however at a lower level such as −10 cmH2O, rather than the traditional −20 cmH2O and in almost all cases the lung will expand (22). Additionally, simple maneuvers such as pulling the chest tube back can lead to stoppage of the air leak. If the chest tube is sitting on the injured lung it may prevent the parenchyma from healing. One last conservative option is to increase the amount of water in the chamber leading to an increased water seal. Increasing the water seal will allow positive pressure from the pleuravac to “push back” on the lung helping the air leak to stop at the moment there is static flow of pressure which will lead to closure of the air leak due to fibrin and platelet deposition.
Fibrin patch
When air leaks persist despite maximal conservative management a good option is the use of fibrin sealants. There are multiple sealants currently on the market and although many are different in what they are derived from (such as totally synthetic, derived from single donor cryoprecipitate or autologous) the mechanism of action remains the same (9-15). They act to simulate the final pathway in the clotting cascade. The products often contain protein concentrate often containing fibrinogen, plasma fibronectin, factor XIII, and plasminogen in an Aprotinin solution is sprayed simultaneously with thrombin in a calcium chloride solution and when combined, the thrombin cleaves fibrinogen into fibrin and a clot is formed at the site of administration (23-25). Aprotinin, an antiproteolytic enzyme, serves to inhibit fibrin degradation to maintain longevity of the sealant (26). More recently, patches soaked in fibrin sealant glue have been used with success to treat PALs in patients (27,28).
Historically the prophylactic use of fibrin sealants intraoperatively and along suture and/staple lanes has been debated. Many authors have studied the efficacy and Fleisher et al. and Wong et al. found that the routine use of fibrin sealants intraoperatively did not significantly shorten the duration of air leak or chest tube length (24,27). More recently, investigators showed that fibrin sealants applied post-operatively via thoracoscopic catheter were able to successfully seal PALs in 24 hours (28). Maximal success was achieved when the fibrin sealant was applied directly onto the air leak site. Similarly, fibrin sealants injected endoscopically via a fiberoptic bronchoscope successfully treated persistent air leaks post-operatively (29).
As with all pharmacologic and medical procedures risks and benefits must always be weighed prior to proceeding. Benefits of fibrin sealants are then are painless and can be administered at the bedside through the already present chest tube (30). Risks of fibrin sealants include the potential for blood borne diseases when using human plasma and potential antigenic stimulant when animal proteins are used (13). Furthermore, fibrin sealants and patches are potential growth media for bacteria and can increase postoperative infection rates (13,26). Advancement in fibrin sealant technology has progressed and the use of synthetic agents and autologous fibrin sealants (9-11,14,15,31) can be safely used in patients to seal PALs, successfully mitigating immune and autoimmune risks.
Doxycycline pleurodesis
For patients with persistent air leaks unresponsive to conservative medical management who are not ideal operative candidates, chemical pleurodesis can be used to treat ongoing PALs and prevent future recurrence (16-18). Chemical sclerosing agents cause fusion of the parietal and visceral pleural layers through inflammation and fibrosis (18). Agents such as doxycycline function through damaging mesothelial surface, interfering with pleural repair, inhibit matrix-degrading metalloproteinases and promote fibrin deposition into the inflamed pleural space (32,33). If the patient is immunosuppressed or on steroids the ability of the inflammatory cascade may be diminished leading to reduced inflammatory reaction to the pleurodesis (34). In traditional methods of chemical pleurodesis, patients are pre-medicated with narcotics before sclerosing agents are introduced via tube thoracostomies (32,33,35). Patients are repositioned continuously every 30 minutes to facilitate distribution of the chemical throughout pleural space.
Despite consensus that chemical pleurodesis is an effective tool in management of PAL for high-risk inoperable patients (8,36), researchers remain divided regarding the best pleural sclerosing agent to use (36-38). Ideal agents should be safe with low adverse effect profiles, inexpensive, and widely available (37). Recent reviews have revealed that the most common agents used by clinicians include talc, tetracycline and derivatives (doxycycline, minocycline), iodopovidone, and silver nitrate (18,35,37,39,40). In 2001, a consensus statement by the American College of Chest Physicians recommended doxycycline as preferred agents for chemical pleurodesis as talc has been associated with ARDS (17). There is one report of acute respiratory failure after pleurodesis with 300mg of doxycycline (41,42). The American Thoracic Society has recommended doxycycline as an efficacious replacement (43).
In making the clinical decision to proceed with chemical pleurodesis, clinicians must be aware of its potential shortcomings. While chemical pleurodesis can be a safe and effective alternative to surgery to prevent future recurrence of air leaks in high-risk patients (8,36), it should only be performed when there is a small or no residual pneumothorax visible on chest radiography (30). Additionally, the high rates of pain reported across a wide range of sclerosing agents (32,33,38,44) indicate that this is not a benign, minimally-invasive procedure. Despite these flaws, chemical pleurodesis, specifically with doxycycline (17), can be an effective tool for treatment of PALS (36).
EBVs
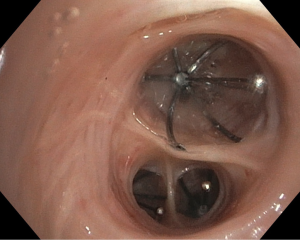
One-way EBVs (Figure 1) are a relatively new advancement in PAL management that provides a safe, minimally-invasive alternative to surgical correction in patients with high surgical risk (45,46). EBVs prevent air from entering pulmonary parenchyma distally while allowing air and secretions to travel proximally and thus be drained (45-47). The occlusion of air to distal lung segments causes isolated atelectasis and effectively removes APLs from the respiratory system (46). It is believed that this controlled atelectasis eventually results in the resolution of air leaks (45). Currently, there are two FDA-approved brands of EBVs available on the market: the IBVTM Spiration made by Olympus and the EBVTM Zephyr (48,49).
The first step in the placement of an EBV is to localize the source of air leak (47). Because multiple bronchial segments can contribute to air leaks, a systematic approach must be taken to identify all APLs. Most often, a Fogarty balloon catheter is used to sequentially occlude branches of bronchus from proximal to distal while observing for cessation of the air leak (45,48,49). Currently, it is recommended that valves be removed after 6 weeks although some authors reported permanently deployed without serious complications (47,50). EBV placement is usually performed with a flexible bronchoscope under conscious sedation (45). Recent advancements in digital air leak quantification technology have allowed surgeons to objectively identify air leaks during EBV placement (51,52). During the sequential occlusion step, a measurement of almost 0 L/min on the digital drainage system allows surgeons to objectively confirm that they have localized the air leak. This is especially helpful when multiple valves are needed.
Although EBVs are still a relatively new technology, few complications have been noted. No mortalities have been reported in the literature and the largest case series (40 patients), performed by Travaline et al., reported six patients with complications after EBV placement; these include valve expectoration, moderate oxygen desaturations, pneumonia, and MRSA colonization (47,53). In studies examining the efficacy of EBV in patients with emphysema, variable success has been seen in the blockage of air to occluded lung segments, resulting in inconsistent amounts of therapeutic atelectasis (54). Such variations are thought to be due to the development of collateral ventilation in patients with chronic emphysema providing persistent airflow to occluded lung segments (54,55). Nevertheless, EBVs have a high success rate in air leak literature, with significant improvement of PALs in 92% of patients and complete resolution in 47.5% in one series (53). Additionally, their ease of placement with flexible bronchoscopy under minimal anesthesia, easy removal, and inability to preclude future surgeries in patients make them a great alternative to surgical intervention in patients with PALs.
Operative intervention
If air leaks persist despite conservative, medical and endoscopic techniques then operative intervention is likely required. Traditionally an open thoracotomy approach was used, but today the mainstay for re-operative intervention is video assisted thoracoscopic surgery (VATS). This is often done through the same incisions the pulmonary resection was initially preformed through assuming it was initially minimally invasive approach. Once the decision is made to take the patient back to the operating room the first step is to identity the air leak and then staple it off. Other techniques include mobilization of the lung for when big spaces exist. Several authors have had good success with pleural tents. Additionally, pleurodesis and use of fibrin sealants can be used intraoperatively to help reduce the incidence of new air leaks at the new staple line.
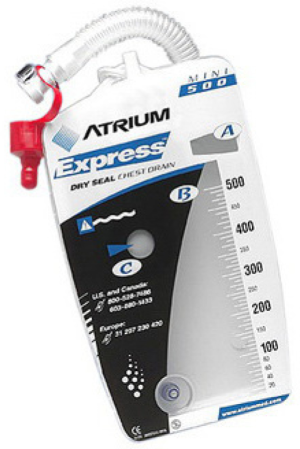
Mini 500
The Mini 500 (Figure 2) is a mobile portable drainage collection unit with pre-set suction to −20 cmH2O. These small devices can drastically increase patient satisfaction as they can leave the hospital while waiting for the air leak to seal on its own. Patients need to be willing and able to care for the pleuravac. When sending a patient home with a pleuravac one can consider the use of antibiotic to cover skin flora and a tube thoracostomy will remain in. Additionally, the surgeon must make sure the lung has re-expanded and is stable on chest X-ray prior to sending patient home.
Conclusions
There are abundant medical and surgical options in the treatment of persistent air leaks. At present there are no current algorithms or set guidelines in the management of PAL. There are several techniques which are popular and often are used based on the clinical judgment and experience of the thoracic surgeon.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-84. [PubMed]
- Cerfolio RJ. Recent advances in the treatment of air leaks. Curr Opin Pulm Med 2005;11:319-23. [Crossref] [PubMed]
- Cerfolio RJ, Bass CS, Pask AH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg 2002;73:1727-30; discussion 1730-1.
- Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg 2003;75:1587-92. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10. [Crossref] [PubMed]
- Chee CB, Abisheganaden J, Yeo JK, et al. Persistent air-leak in spontaneous pneumothorax--clinical course and outcome. Respir Med 1998;92:757-61. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206; discussion 206-7. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after ma-jor pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Gonfiotti A, Santini P, Jaus M, et al. Safety and effectiveness of a new fibrin pleural air leak sealant: A multicenter, controlled, prospective, parallel-group, randomized clinical trial. Ann Thorac Surg 2011;92:1217-24; discussion 1224-5. [Crossref] [PubMed]
- Kawamura M, Gika M, Izumi Y, et al. The sealing effect of fibrin glue against alveolar air leakage evaluated up to 48h; comparison between different methods of application. Eur J Cardiothorac Surg 2005;28:39-42. [Crossref] [PubMed]
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8. [Crossref] [PubMed]
- Matar AF, Hill JG, Duncan W, et al. Use of biological glue to control pulmonary air leaks. Thorax 1990;45:670-4. [Crossref] [PubMed]
- Matthew TL, Spotnitz WD, Kron IL, et al. Four years’ experience with fibrin sealant in thoracic and cardiovascular surgery. Ann Thorac Surg 1990;50:40-3. [Crossref] [PubMed]
- Robinson CL. Autologous blood for pleurodesis in recurrent and chronic spontaneous pneumothorax. Can J Surg 1987;30:428-9; discussion 43-4. [PubMed]
- Moser C, Opitz I, Zhai W, et al. Autologous fibrin sealant reduces the incidence of prolonged air leak and duration of chest tube drainage after lung volume reduction surgery: A prospective randomized blinded study. J Thorac Cardiovasc Surg 2008;136:843-9. [Crossref] [PubMed]
- Almassi GH, Haasler GB. Chemical pleurodesis in the presence of persistent air leak. Ann Thorac Surg 1989;47:786-7. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: An American college of chest physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- How CH, Hsu HH, Chen JS. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc 2013;112:749-55. [Crossref] [PubMed]
- Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112:1319-29; discussion 1329-30. [Crossref] [PubMed]
- Cooper JD, Patterson GA. Lung-volume reduction surgery for severe emphysema. Chest Surg Clin N Am 1995;5:815-31. [PubMed]
- Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. [Crossref] [PubMed]
- Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 2002;121:831-5. [Crossref] [PubMed]
- Wong K, Goldstraw P. Effect of fibrin glue in the reduction of postthoracotomy alveolar air leak. Ann Thorac Surg 1997;64:979-81. [Crossref] [PubMed]
- Sierra DH. Fibrin sealant adhesive systems: A review of their chemistry, material properties and clinical applications. J Biomater Appl 1993;7:309-52. [Crossref] [PubMed]
- Fleisher AG, Evans KG, Nelems B, et al. Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg 1990;49:133-4. [Crossref] [PubMed]
- Miyamoto H, Futagawa T, Wang Z, et al. Fibrin glue and bioabsorbable felt patch for intraoperative in-tractable air leaks. Jpn J Thorac Cardiovasc Surg 2003;51:232-6. [Crossref] [PubMed]
- Lopez C, Facciolo F, Lequaglie C, et al. Efficacy and safety of fibrin sealant patch in the treatment of air leakage in thoracic surgery. Minerva Chir 2013;68:559-67. [PubMed]
- Thistlethwaite PA, Luketich JD, Ferson PF, et al. Ablation of persistent air leaks after thoracic procedures with fibrin sealant. Ann Thorac Surg 1999;67:575-7. [Crossref] [PubMed]
- Finch CK, Pittman AL. Use of fibrin glue to treat a persistent pneumothorax with bronchopleural fistula. Am J Health-Syst Pharm 2008;65:322-4. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of persistent air leaks. Chest 2017;152:417-23. [Crossref] [PubMed]
- Iyama S, Sato T, Murase K, et al. Successful treatment of fibrin glue sealant for pneumothorax with chronic GVHD resistant to autologous blood patch pleurodesis. Intern Med 2012;51:2011-4. [Crossref] [PubMed]
- Hatata E, Daabis R, Sabaa M, et al. Doxycycline poudrage: An old agent for a new technique. Egypt J Chest Dis Tuberc 2017;66:331-8. [Crossref]
- Heffner JE, Standerfer RJ, Torstveit J, et al. Clinical efficacy of doxycycline for pleurodesis. Chest 1994;105:1743-7. [Crossref] [PubMed]
- Teixeira LR, Wu W, Chang DS, et al. The effect of corticosteroids on pleurodesis induced by doxycy-cline in rabbits. Chest 2002;121:216-9. [Crossref] [PubMed]
- Jabłoński S, Kordiak J, Wcisło S, et al. Outcome of pleurodesis using different agents in management prolonged air leakage following lung resection. Clin Respir J 2018;12:183-92. [Crossref] [PubMed]
- Hallifax RJ, Yousuf A, Jones HE, et al. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: A systematic review. Thorax 2017;72:1121-31. [Crossref] [PubMed]
- Tabatabaei SA, Hashemi SM, Kamali A. Silver nitrate versus tetracycline in pleurodesis for malignant pleural effusions; a prospective randomized trial. Adv Biomed Res 2015;4:178. [PubMed]
- Song KS, Keum D, Kim JB. Chemical pleurodesis using doxycycline and viscum album extract. Korean J Thorac Cardiovasc Surg 2017;50:281-6. [Crossref] [PubMed]
- Baumann MH, Strange C. The clinician’s perspective on pneumothorax management. Chest 1997;112:822-8. [Crossref] [PubMed]
- Elnady M, Sakr A. Safety and efficacy of pleurodesis with thoracoscopic doxycycline poudrage in malignant pleural effusion. Eur Respir J 2011;38:55.
- Baumann MH. Treatment of spontaneous pneumothorax. Curr Opin Pulm Med 2000;6:275-80. [Crossref] [PubMed]
- DiBardino DJ, Vanatta JM, Fagan SP, et al. Acute respiratory failure after pleurodesis with doxycycline. Ann Thorac Surg 2002;74:257-8. [Crossref] [PubMed]
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- How CH, Tsai TM, Kuo SW, et al. Chemical pleurodesis for prolonged postoperative air leak in primary spontaneous pneumothorax. J Formos Med Assoc 2014;113:284-90. [Crossref] [PubMed]
- Gkegkes ID, Mourtarakos S, Gakidis I. Endobrachial valves in treatment of persistent air leaks: A systematic review of clinical evidence. Med Sci Monit 2015;21:432-8. [Crossref] [PubMed]
- Hance JM, Martin JT, Mullett TW. Endobronchial valves in the treatment of persistent air leaks. Ann Thorac Surg 2015;100:1780-5; discussion 1785-6.
- Lazarus DR, Casal RF. Persistent air leaks: A review with an emphasis on bronchoscopic management. J Thorac Dis 2017;9:4660-70. [Crossref] [PubMed]
- Cordovilla R, Torracchi A, Novoa N, et al. Endobrachial valves in the treatment of persistent air leak, an alternative to surgery. Arch Bronconeumol 2015;51:10-5. [PubMed]
- Ding M, Gao Y, Zeng X, et al. Endobronchial one-way valves for treatment of persistent air leaks: A systematic review. Respir Res 2017;18:186-96. [Crossref] [PubMed]
- Fiorelli A, Andrilli A, Cascone R, et al. Unidirectional endobronchial valves for management of persistent air-leaks: Results of a multicenter study. J Thorac Dis 2018;10:6158-67. [Crossref] [PubMed]
- Zisis C, Tsirgogianni K, Lazaridis G, et al. Chest drainage systems in use. Ann Transl Med 2015;3:43. [PubMed]
- Saettele TM, Jimenez CA. Digital quantification of air leak to identify the location of an alveolopleural fistula. Ann Am Thorac Soc 2014;11:1152-4. [Crossref] [PubMed]
- Travaline JM, McKenna RJ Jr, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax 2006;61:371-3. [Crossref] [PubMed]
- Klooster K, Hacken N, Hartman J, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Atrium Medical Express Mini 500 Chest Drain, Disposable. Bound Tree. Available online: www.boundtree.com/Suction/Suction-Units/Atrium-Medical-Express-Mini-500-Chest-Drain,-Disposable/p/660865