
Role of bronchoscopy in management of central squamous cell lung carcinoma in situ
Introduction
Carcinoma in situ (CIS) is the preinvasive stage of lung cancer without any evidence of metastatic spread. Squamous cell carcinoma in situ (SCIS) is superficial and without any involvement of the basement membrane. It can be detected in the central airways and is amenable to therapeutic bronchoscopic interventions. Microinvasive carcinoma (MIC) invades the basement membrane but spares the cartilage. In contrast, adenocarcinoma in situ (AIS), the preinvasive form of adenocarcinoma is mostly located in the lung parenchyma and is usually diagnosed on resected specimen. Recent guidelines have provided a clearer definition of AIS with dimensions of <3 cm, lepidic pattern and lack of invasion (1,2). SCIS is not defined by any size criteria of the superficial lesion and can be difficult to differentiate from high grade dysplasia even for experienced pathologists (3).
Squamous cell carcinomas progress from premalignant lesions (PML) namely basal cell hyperplasia, to metaplasia, to dysplasia, to CIS and then to invasive disease (2,4). Squamous dysplasia may be low grade (mild and moderate dysplasia) or high grade (severe dysplasia), with severity of dysplasia based on progressive cytological abnormalities, loss of maturation and increasing degree of involvement of the thickness of the epithelium (5). SCIS has cytological aberrations, uneven chromatin, mitoses occurring at all levels, and absent maturation with sparing of the basement membrane (2,5). Not all SCIS progresses to invasive disease and almost one third to half may either regress or remain stable (6,7). Genetic studies have shed light on the profile of these lesions and may help in predicting which will progress to invasive disease (6,8). Driver mutation alterations in TP53, CDKN2A, SOX2, and AKT2, and less frequent alterations in FAT1, KMT2D, KEAP1, EGFR, and NOTCH1 have been described in SCIS lesions. Distal chromosome 3q in the region containing the gene ECT2, a regulator of cytokinesis that is associated with chromosomal instability is associated with development of invasive disease (6). Some of the driver genes involved in invasive disease include TPM3, PTPRB, SLC34A2, KEAP1, NKX2-1, SMAD4, and SMARCA4, homeobox family (HOXC8, HOXC9, HOXC10, HOXD10, HOXA11AS) (6). Although above research findings have enhanced our knowledge of PML’s, clinically it is still unclear as to which lesions will stay stable, which will step wise progress to SCIS and which will directly progress to invasive carcinoma although the presence of these PML’s indicates an overall higher risk of lung cancer based on the theory of “field carcinogenesis” (9). With a better understanding of what causes abnormal cells to turn into invasive disease lies the opportunity to develop better prevention, screening, diagnostic and treatment strategies for lung cancer.
Diagnosis
Centrally located SCIS is usually not detected on routine CT of the chest (10). The patients are generally asymptomatic and SCIS is mostly diagnosed incidentally on bronchoscopy as thickened, nodular or polypoid lesions although unusual cases of airway obstruction by SCIS have been reported (11-13). Sputum cytology and routine white light bronchoscopy (WLB) have poor diagnostic yields for SCIS (10). SCIS has understandably a much better prognosis and therefore early detection is the key to successful management (10). As patients with SCIS tend to have synchronous lesion, limited lung reserve and multiple co-morbidities surgical resection is not always feasible. For patients who are not surgical candidates, current guidelines recommend endobronchial treatment (14).
Role of bronchoscopy in diagnosis SCIS
SCIS can be detected incidentally during routine WLB or with advanced bronchoscopic techniques like autofluorescence bronchoscopy (AFB), narrow band imaging (NBI), high magnification bronchoscopy (HMB) and high definition (HD) bronchoscopy (15). The abnormal areas may be further evaluated with endobronchial ultrasound (EBUS), optical coherence tomography (OCT), Confocal laser endomicroscopy and laser Raman spectroscopy (LRS) (15). Other than AFB and NBI the data on the other techniques in SCIS is extremely limited.
AFB
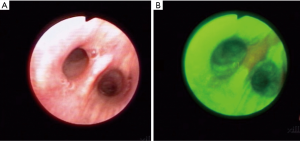
AFB is based on the principle that normal bronchial tissue has fluorophores and it exhibits fluorescence when exposed to light of certain wavelength and this characteristic is altered by cancer. Several bronchoscopic systems use this principle to differentiate normal from abnormal mucosa by using blue light for airway illumination and determining the tissue fluorescence by capturing images in red and green wavelength range. In AFB, normal bronchial tissues fluoresces strongly in the green, whereas premalignant and malignant tissues have a much lower green autofluorescence and may appear as shades of red, brown or magenta depending on the AFB system used (Figure 1) (15). This ability to differentiate normal from abnormal epithelium generated significant interest in this technology with several publications comparing it with sputum and WLB and some studies even attempted bimodality screening of AFB with low dose CT (LDCT) chest (14,16). Overall these studies reveal that AFB has increased sensitivity as compared to WLB in the diagnosis of SCIS, however, the specificity is lower. Non-specific inflammation in the airways also looks abnormal and this decreases the specificity of AFB and leads to additional biopsies and results in higher cost. Recent data from Pan-Canadian early detection of lung cancer study revealed low detection rate of SCIS with AFB, thus discouraging its use as a tool for lung cancer screening (17). In this study, AFB was performed along with LDCT on 1,300 participants who had an estimated 2% or greater lung cancer risk over 5 years. LDCT detected 56 prevalent lung cancers (4.3%) while AFB detected only 5 cases after 776 endobronchial biopsies from 333 patients. Of these 5 cases only 2 were occult on LDCT (one SCIS and one carcinoid) with the incremental value of AFB added to LDCT of only two out of 1,300 or 0.15% (95% CI, 0.0–0.6%). The reason for this low yield of AFB was that over 90% of lung cancers did not arise in the central airways that are reachable by routine bronchoscopy (18). Recent trends indeed have shown a declining incidence of squamous cell cancer in favor of increasing frequency of adenocarcinoma (19) and higher rate of peripheral instead of central squamous cell lung cancers (20). Although AFB may not be helpful as a screening tool, it still may have important role in the research aimed at understanding the process of airway carcinogenesis and studies of chemoprevention (8,21). With development of thin and ultrathin bronchoscopes, perhaps an AFB system that can access distal airways may be more beneficial.
NBI
NBI is an image-enhanced bronchoscopic technique that uses narrow-band range filters to enhance the visualization of mucosal and submucosal vessels thus allowing the assessment of abnormal angiogenesis which is observed in premalignant and malignant lesions (10). NBI uses 2 narrow bands of light one being blue (390–445 nm) and other being green (530–600 nm) (15). These two wavelengths match the two absorption peaks of hemoglobin thus allowing an enhanced visualization of the blood vessels (22). The blue light has a shorter wavelength and thus has less penetrance and less scattering and is helpful in visualization of superficial capillaries while the green light aids in visualization of the deeper submucosal veins (Figure 2). Abnormal vascular patters include increased vessel growth, tortuous vessels, dotted vessel and spiral or screw type vessels (23).
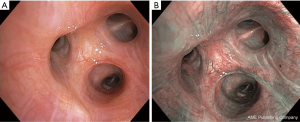
A recent metanalysis concluded that NBI has higher sensitivity, specificity and diagnostic odds ratio as compared to AFB for PML (24). Smaller studies have suggested a role of NBI and AFB in improved assessment of extent of SCIS (25,26). The NBI vascular pattern may even be helpful in assessing the histopathology of lung cancer with dotted vessels being more commonly seen in adenocarcinoma and tortuous and abrupt ending vessels being more frequent in squamous cell carcinoma (27).
OCT
OCT uses near infra-red (IR) light which interacts with tissue architecture as a function of depth (similar to ultrasound but without needing a fluid medium) and allows for the creation of near-histologic cross section images through optical interferometry (28). The spatial resolution of OCT is 3 to 15 µm and a depth of penetration of 2 mm (15). OCT can be performed with a small probe that can pass through the working channel of a bronchoscope. Quantitative measurement of epithelial thickness and intrusion of basement membrane may be helpful in distinguishing dysplasia, SCIS and invasive carcinoma (28). Due to enormous amount of data that could be generated from microscopic level resolution of OCT, evaluation of the entire airway may not be feasible. It may be prudent to use it as a complementary tool to AFB or NBI to focus on an abnormal area and help improve the specificity of these techniques.
Radial probe endobronchial ultrasound (RP-EBUS)
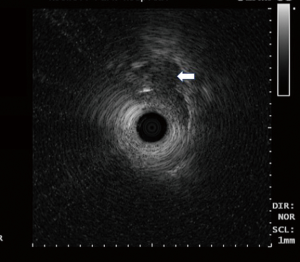
RP-EBUS uses a 20 MHz rotating probe that is capable of producing 360° circumferential images in a direction perpendicular to the axis of the probe with a resolution of <1 mm and a penetration depth of 5 cm (Figure 3) (29,30). The RP-EBUS with balloon (UM-BS20-26R, Olympus, Japan) can illustrate three to seven echo layers of the airway wall (29). Kurimoto et al. used RP-EBUS with balloon probe on surgically removed trachea and bronchi and were able to accurately predict the depth of invasion of the tracheobronchial wall in 23 of 24 cases of lung cancer (31). Few additional small clinical studies have utilized balloon filled RP-EBUS in determining the depth of tumor invasion and aiding in appropriate therapeutic decisions (32-35).
Other bronchoscopic modalities
HD bronchoscopy provides HD detailed images of the bronchial surface. The latest bronchoscopy systems are mostly HD capable. HMB has a video observation system that can provide four-fold high magnification of a target area as compared to conventional bronchoscope, thus providing a view of the microvascular structure (15,36). Probe based confocal laser endomicroscopy uses confocal fluorescent microscopy to provide in vivo imaging below the bronchial surface with detailed analysis of cellular and subcellular structures which may aid in evaluation of the neoplastic changes (15,37). LRS focuses a low-power laser light to the bronchial tissue to collect scattered light for spectroscopic analyses (15). These are encouraging advances in bronchoscopy which need further evidence to support their role in management of SCIS.
Treatment
Bronchoscopy plays a key role not only in the diagnosis of the central SCIS but also in the staging, management and follow up of these lesions. Bronchoscopy enables minimally invasive and safer management of SCIS as compared to surgery and external beam radiation. AFB and NBI can accurately determine the true size of the lesion as compared to the WLB. Linear probe EBUS (LP-EBUS) may be useful in sampling adjacent lymph nodes if there is any question of local lymph node involvement and RP-EBUS can look for evidence of invasion of the cartilage and deeper structures of the airway wall. Once the lesions are appropriately evaluated, the next step is to decide if the SCIS is amenable to bronchoscopic ablation and the following techniques are helpful in achieving this.
Photodynamic therapy (PDT)
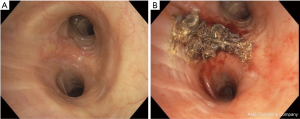
PDT works by the interaction of light, a photosensitizer (PS) drug and oxygen (38,39). A tumor specific PS is injected into the body which gets concentrated into the tumor tissue. At a specific time range after the injection of the PS, when it has concentrated in the target tissue, exposure to light of specific wavelength results in generation of reactive oxygen species (ROS), especially singlet oxygen radicals, which affect all the intracellular components, including proteins and DNA, resulting in necrosis or apoptosis. Along with direct cell killing and apoptosis, PDT also destroys tumor vasculature, and induces an inflammatory reaction that leads to a congregation of anti-tumor immune responses (39,40). Cells with intracellular PS are selectively damaged, and the surrounding tissue is spared. This tumor specificity is helpful in SCIS of the central airways as occasionally the margins of the lesions are not well-defined. Because the PS is also retained in other organs including the skin, patients may get a sunburn after light exposure. Therefore, light precautions need to be initiated for a time range which is specific for each PS. Patients may need clean up bronchoscopies after treatment to remove debris (Figure 4).
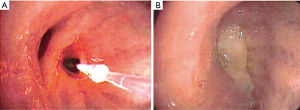
PDT has been extensively studied for bronchoscopic ablation of CIS. The most commonly used PS in the Unites States is Porfimer sodium (Photofrin, Pinnacle Biologics, USA) which works with 630 nm red-light irradiation. Photofrin is associated with significant incidence of sunburn in 13–41% cases and patients need to avoid sunlight for 4–6 weeks post treatment (41). Most cases of photosensitivity are due to non-compliance with light precautions. Second generation PS drugs with deeper penetration and less photosensitivity are now being evaluated.
In one of the earliest report from 1982, hematoporphyrin derivative (HpD) was utilized for treating lung cancer with PDT (42). HpD was activated by visible red light (630 nm, 90 to 400 mW) with an argon dye laser. Out of 13 cases of lung cancer one patient had two small (size 2 mm each) early stage lung cancer lesions which achieved complete remission (CR) after PDT. This generated an interest in PDT and subsequently several additional studies described the role of PDT in early stage lung cancer.
A phase II study of PDT with porfimer sodium for centrally located early-stage lung cancer reported a CR of 84.7% (50/59 lesions) after initial treatment (43). The median duration of CR was 14 months. The response was better for lesions that were smaller. Of the 45 carcinomas that had a longitudinal tumor extent <1 cm, 44 (97.8%) had a CR while out of the 14 carcinomas with a longitudinal tumor extent of >1 cm, only 6 (42.9%) showed a CR (P=0.00001). Multiple regression analysis showed that the estimated longitudinal extent of the tumor was the only independent prognostic indicator. Other authors have also shown the decrease of CR rate as the size of lesions increased with a CR of 94.6% of lesions <5 mm, 93.5% for 0.5–1.0 cm, 80% for 1–2 cm and only 44.1% for >2.0 cm (44). In fact, other bronchoscopy-surgical histopathology correlation studies based on surgical resection specimens have demonstrated a lesser likelihood of deeper invasion in lesions <1 cm (45,46).
One more study of porfimer sodium on 93 patients with 114 central early-stage lung cancers reported similar findings with a higher complete response for lesions <1.0 cm (77/83) as compared to lesions ≥1.0 cm (18/31) [93% vs. 58%, P<0.001]. The authors performed bronchoscopic measurements of the tumor size with forceps and obtained biopsies of the proximal and distal end of the lesion for getting accurate measurements of the superficial extent of the lesions. Out of the 77 patients with <1.0 cm lesion who achieved CR, 9 patients developed recurrence on follow up bronchoscopy. This was attributed to an inappropriate estimation of peripheral margins or depth of the lesions. It was realized that even lesions <1.0 cm can have deeper invasion. The authors suggested AFB to determine the superficial extent of the disease and RP-EBUS to assess the depth of invasion before treating with PDT. By this time, some other reports were already describing the utility of RP-EBUS for determining cartilage invasion and selecting appropriate candidates for PDT (33,35). Above studies suggest that smaller lesions without deeper invasion respond well to PDT and all patients who undergo definitive PDT should be carefully monitored for local recurrence.
Progress has been made with the development of newer agents with better tissue penetration and less side effects than porfimer sodium. Second generation photo sensitizers including 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) and mono-L-aspartyl chlorine e6 (NPe6, talaporfin sodium, laserphyrin) are being studied as they cause less photosensitivity than photfrin (12,47,48).
A phase 1 study of a new PS HPPH for SCIS and MIC showed a CR of 82.4% (14/17 lesions) at one-month and 72.7% (8/11 lesions) at 6 months. One patient with lack of response was found to have cartilage invasion by RP-EBUS after treatment (the study institution procured the RP-EBUS technology after PDT treatment of this patient with HPPH). HPPH has deeper penetration due to a longer absorption peak of 665 nm as compared to 630 nm for porfimer sodium and it has lower photosensitivity rate and sunlight precautions are only needed for 7–10 days (47).
Another second-generation PS, NPe6 (talaporfin sodium, laserphyrin) has been studied and approved in Japan for SCIS. The compound also has deeper penetration as it has a major absorption peak at 664 nm. Laser light excitation is done after 4–6 hours of intravenous injection. A phase 1 study of CIS and early invasive cancer <2 cm showed a CR of 84.6% (33/39 lesions) (49). Photosensitivity rate was low with grade 1 toxicity in 10% and no case of grade 2–4 toxicity. Light precautions were only needed for 2 weeks. The same group reported a CR rate of 92.1% (35/38 lesions) in another study of talaporfin in which AFB (SAFE 3000, Pentax, Tokyo) along with RP-EBUS and OCT were utilized to define the extent of the tumor (12). The authors described using a photodynamic diagnostic system (PDD) using the SAFE 3000 AFB system which helped them define the lesion in a better fashion. A loss of red fluorescence on AFB was seen immediately after PDT suggesting adequate treatment. It is to be noted that patients were hospitalized for 7–10 days after treatment. A third study by the same group using talaporfin for even lesions >1.0 cm reported a high CR of 90.5% (19/21 lesions) (13). They used OCT to determine invasion of the cartilage and claimed success even in cases of cartilage invasion due to deeper penetration of talaporfin. Another interesting aspect of this study was that some larger lesions were debulked with electrocautery before being successfully treated with PDT with clinical success. Although these outcomes need confirmation in additional trials, these findings have opened a new door for PDT use in central airway cancers.
In summary, PDT is the most well studied bronchoscopic treatment for SCIS. Although initially limited to very small superficial visible lesions, the new agents may broaden the application for larger and deeper lesions. The use of newer technologies like RP-EBUS and OCT have allowed for a better assessment of these lesions and hopefully will be incorporated into protocols of new studies. Photosensitivity, frequent bronchoscopies for removal of debris, need of hospitalization and limited availability are the major drawbacks of this technology.
Endobronchial brachytherapy (EBBT)
EBBT causes single chain damage to DNA resulting in apoptosis rather than directly killing cells. EBBT is the technique of applying local radiation to the endoluminal surface. Using flexible bronchoscopy, a probe with dummy seeds is placed in the desired location. Once secured in an appropriate location, the dummy seeds are removed and the radiation source (usually Iridium-192) is advanced to the desired position under computer control using a remote after loading unit. Computer aided intended dose distribution is achieved by altering the position of the radiation source and tissue exposure time (50). High dose rate (HDR) is generally preferred over more frequent lower dose treatments. EBBT has the deepest tissue penetration as compared to other bronchoscopic modalities and can destroy tumor in peri-bronchial tissue and the outside cartilage layer (50). Fatal hemoptysis and bronchial necrosis with fistula formation are major complications specially in patients who have previously received external beam radiation or tumor location in upper lobe bronchus close to the major vessels (51). Bronchitis, radiation pneumonitis and bronchial stenosis are other known complications (51). Initially EBBT was described for palliation of large endobronchial tumors and later for early stage lung cancer which included some patients with SCIS (52-59).
Hennequin et al. described treatment with multiple fractions of HDR EBBT using iridium-192 in 106 patients with limited endobronchial disease, defined as bronchoscopically accessible lung carcinoma, <1 cm thick or not visible on CT and without any evidence of metastasis (54). Five of these patents had in situ carcinoma. Complete histologic response rate was 59.4% at 3 months and 51.6% at 5 years. Five deaths were reported in this group, two from fatal hemoptysis and three from bronchial necrosis. Kawamura et al. reported HDR EBBT in 13 patients with 16 lesions including 7 roentgenographically occult lesions (53). Ten lesions were also given external beam radiation. The two-year local control rates of brachytherapy alone and combination of brachytherapy and external beam radiation were 80.0% and 88.9%, respectively. Although all patients had inflammation and telangiectasia, only 15% had dyspnea/chronic cough related to the treatment. Pérol et al. used HDR EBBT on 19 patients with proximal non-small cell lung cancer with maximal diameter <1 cm, not visible on CT and who were not candidates for other treatment like surgery or external beam radiation therapy due to limited respiratory reserve (56). They achieved an 83% local control at 2 months and 75% at 12 months. Unfortunately, even in this small group, one patient with local control died from hemoptysis 12 months after treatment and two patients developed severe necrosis of bronchial wall with one of them dying from hemoptysis.
In summary, there are no well-designed studies of HDR EBBT in SCIS and the rate of severe complications is a concern. It is possible that the source-delivering catheter is not in the center of the bronchial lumen thus delivering uneven radiation to the airway wall resulting in local complications. The use of devices to keep the catheter in the center of the lumen to avoid eccentric position may reduce some complications (58).
Bronchoscopic cryotherapy
Cryotherapy causes tumor destruction due to the cytotoxic effect of cold on tissue. The application of an extremely cold probe results in formation of ice crystals. The intracellular crystals damage the cell organelles especially the mitochondria and the extra-cellular crystals cause cellular dehydration (50). Vascular thrombosis results as a delayed effect. Cycles of rapid cooling with slow thawing promote cytotoxicity. The depth of treatment is around 3 mm (50). Cartilage, collagen and poorly vascular tissues are is not affected and so this technique works well only for superficial cancers and there is no risk of airway wall perforation or stenosis (50).
Cryotherapy can be administered via flexible or rigid bronchoscope using appropriate probes. It works based on the Joules-Thompson effect (sudden expansion of gas when it moves from high to low pressure zone causes cooling). Nitrous oxide is the most widely used cryogen through flexible cryoprobes with 1.9 or 2.4 mm diameters and the probe tip typically achieve a temperature of 40 °C. The cryoprobe is placed in contact with the tumor and multiple cycles of freezing and thawing are applied over the whole surface of the tumor. Around 7–10 days after treatment, the necrotic debris are removed with bronchoscopy. Cryotherapy is considered a very safe therapeutic modality with side effects including transient fever and sometimes symptoms related to obstructive debris.
Despite being an appealing treatment option there is very limited published data on the use of endobronchial cryotherapy of SCIS. Deygas et al. used cryotherapy through a rigid bronchoscope in 35 patients (41 lesions) with early superficial bronchogenic carcinoma (60). They achieved a CR of 91.4% (32/35 patients) at 1 year with no serious adverse effects. Almost a third of patients had local recurrence within 4 years with many developing invasive carcinomas. Just like most of the other studies, depth of invasion was not evaluated in this study before cryotherapy treatment. In summary, cryotherapy is an attractive and safe option for treatment of SCIS and hopefully this modality will be supported by additional data with well-done clinical studies.
Electrocautery/argon plasma coagulation (APC)
Electrocautery uses electric current and APC uses ionised Argon jet flow (plasma) for thermal destruction of tumors (Figure 5). APC can be used around corners. Again, data on the use of these techniques in the management of SCIS is very limited. One prospective case series has shown electrocautery to be effective in curative treatment for radiographically occult early lung cancer in 13 patients with 80% CR (12/15 lesions) (61). Fibreoptic bronchoscopy under local anaesthesia was used to coagulate the tumor using 30 watt of energy which was applied until visible necrosis of the tumour was seen. Unlike lasers, these two thermal techniques are relatively easily available and are cost-effective.
Light amplification by stimulated emission of radiation (laser)
Laser light can be delivered through optical fibers and can cause tissue destruction due to heat or by photochemical effect (as described in PDT) (62,63). A variety of thermal laser systems are available including neodynium-yttrium aluminum garnet (Nd-YAG) and neodymium: yttrium aluminium pevroskite (Nd:YAP), which are more frequently used with bronchoscopy. The depth of treatment ranges from 0.5–15 mm depending on the type of laser and wattage used (63). Complications include hypoxemia, endobronchial fire, hemorrhage, perforation, stenosis and gas embolism (63). Laser precautions need to be instituted to prevent injury to the patient and the team. Selective use of lasers have been described in manuscripts for management of early stage cancer especially for recurrences after intended study treatment (59,64), but to the best of our knowledge a dedicated study in the scenario of SCIS has not been published. In one of the frequently quoted large series of bronchoscopic laser on 1,585 patients, Cavaliere et al. briefly described use of Nd-YAG laser in nineteen bronchogenic CIS without any recurrence (65). Due to the high risk of perforation the use of Laser in SCIS is discouraged (66).
Mechanical debulking
The need for treatment of CIS has been somewhat controversial as some CIS lesions may regress. In a comprehensive follow up study of PML using AFB guided biopsies, it was shown that 21.9% (7/32) CIS lesions improved spontaneously with 2 becoming moderate dysplasia and 5 becoming even lower grade or normal (7). Overall 78% of the lesions remained CIS/severe dysplasia at 3 months and were treated and the authors suggested that overall CIS should generally be treated. It is likely that the biopsy procedure itself may remove the cancer as illustrated by few case-reports and may an impression of spontaneous regression (67,68). Endobronchial biopsy may therefore be an option for well-defined small lesions that can be completely removed with forceps.
Conclusions
Bronchoscopy has played a vital role in furthering our understanding of the evolution of PML’s and diagnosis and management of SCIS/MIC. Advancement in bronchoscopic imaging modalities can now help in evaluating the extent of the disease which can aid in selection of appropriate management strategy. This can reduced the recurrence rate observed in previous studies due to inclusion of invasive cancer and ultimately bronchoscopy may replace surgery as first line recommended therapy for central SCIS. Hopefully future clinical trials will incorporate these technological advances and shed more light on the appropriate treatment and surveillance of these lesions.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Nicholson AG, Perry LJ, Cury PM, et al. Reproducibility of the WHO/IASLC grading system for pre-invasive squamous lesions of the bronchus: a study of inter-observer and intra-observer variation. Histopathology 2001;38:202-8. [Crossref] [PubMed]
- Banerjee AK. Preinvasive lesions of the bronchus. J Thorac Oncol 2009;4:545-51. [Crossref] [PubMed]
- Thakrar RM, Pennycuick A, Borg E, et al. Preinvasive disease of the airway. Cancer Treat Rev 2017;58:77-90. [Crossref] [PubMed]
- Teixeira VH, Pipinikas CP, Pennycuick A, et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat Med 2019;25:517-25. [Crossref] [PubMed]
- Bota S, Auliac JB, Paris C, et al. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med 2001;164:1688-93. [Crossref] [PubMed]
- Campbell JD, Mazzilli SA, Reid ME, et al. The Case for a Pre-Cancer Genome Atlas (PCGA). Cancer Prev Res (Phila) 2016;9:119-24. [Crossref] [PubMed]
- van Boerdonk RA, Smesseim I, Heideman DA, et al. Close Surveillance with Long-Term Follow-up of Subjects with Preinvasive Endobronchial Lesions. Am J Respir Crit Care Med 2015;192:1483-9. [Crossref] [PubMed]
- Daniels JM, Sutedja TG. Detection and minimally invasive treatment of early squamous lung cancer. Ther Adv Med Oncol 2013;5:235-48. [Crossref] [PubMed]
- Coiffard B, Laroumagne S, Plojoux J, et al. Minimally Invasive but Maximally Obstructive: Carcinoma In Situ Obstructing a Mainstem Bronchus. J Bronchology Interv Pulmonol 2017;24:67-9. [Crossref] [PubMed]
- Usuda J, Tsutsui H, Honda H, et al. Photodynamic therapy for lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy. Lung Cancer 2007;58:317-23. [Crossref] [PubMed]
- Usuda J, Ichinose S, Ishizumi T, et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clin Cancer Res 2010;16:2198-204. [Crossref] [PubMed]
- Wisnivesky JP, Yung RC, Mathur PN, et al. Diagnosis and treatment of bronchial intraepithelial neoplasia and early lung cancer of the central airways: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e263-77S.
- Inage T, Nakajima T, Yoshino I, et al. Early Lung Cancer Detection. Clin Chest Med 2018;39:45-55. [Crossref] [PubMed]
- Loewen G, Natarajan N, Tan D, et al. Autofluorescence bronchoscopy for lung cancer surveillance based on risk assessment. Thorax 2007;62:335-40. [Crossref] [PubMed]
- Tremblay A, Taghizadeh N, McWilliams AM, et al. Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. Chest 2016;150:1015-22. [Crossref] [PubMed]
- Ost DE. The Importance of Negative Studies: Autofluorescence Bronchoscopy for Lung Cancer Screening. Chest 2016;150:993-4. [Crossref] [PubMed]
- Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13-22. [Crossref] [PubMed]
- Drilon A, Rekhtman N, Ladanyi M, et al. Squamous-cell carcinomas of the lung: emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol 2012;13:e418-26. [Crossref] [PubMed]
- Beane J, Mazzilli SA, Tassinari AM, et al. Detecting the Presence and Progression of Premalignant Lung Lesions via Airway Gene Expression. Clin Cancer Res 2017;23:5091-100. [Crossref] [PubMed]
- Gono K. Narrow Band Imaging: Technology Basis and Research and Development History. Clin Endosc 2015;48:476-80. [Crossref] [PubMed]
- Shibuya K, Nakajima T, Fujiwara T, et al. Narrow band imaging with high-resolution bronchovideoscopy: a new approach for visualizing angiogenesis in squamous cell carcinoma of the lung. Lung Cancer 2010;69:194-202. [Crossref] [PubMed]
- Iftikhar IH, Musani AI. Narrow-band imaging bronchoscopy in the detection of premalignant airway lesions: a meta-analysis of diagnostic test accuracy. Ther Adv Respir Dis 2015;9:207-16. [Crossref] [PubMed]
- Zaric B, Becker HD, Perin B, et al. Narrow band imaging videobronchoscopy improves assessment of lung cancer extension and influences therapeutic strategy. Jpn J Clin Oncol 2009;39:657-63. [Crossref] [PubMed]
- Zaric B, Perin B, Becker HD, et al. Combination of narrow band imaging (NBI) and autofluorescence imaging (AFI) videobronchoscopy in endoscopic assessment of lung cancer extension. Med Oncol 2012;29:1638-42. [Crossref] [PubMed]
- Zaric B, Perin B, Stojsic V, et al. Relation between vascular patterns visualized by Narrow Band Imaging (NBI) videobronchoscopy and histological type of lung cancer. Med Oncol 2013;30:374. [Crossref] [PubMed]
- Lam S, Standish B, Baldwin C, et al. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res 2008;14:2006-11. [Crossref] [PubMed]
- Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc 2009;6:180-6. [Crossref] [PubMed]
- Andolfi M, Potenza R, Capozzi R, et al. The role of bronchoscopy in the diagnosis of early lung cancer: a review. J Thorac Dis 2016;8:3329-37. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of Usefulness of Endobronchial Ultrasonography in Determination of Depth of Tracheobronchial Tumor Invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
- Miyazu Y, Miyazawa T, Kurimoto N, et al. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832-7. [Crossref] [PubMed]
- Li J, Chen PP, Huang Y, et al. Radial probe endobronchial ultrasound scanning assessing invasive depth of central lesions in tracheobronchial wall. Chin Med J (Engl) 2012;125:3008-14. [PubMed]
- Takahashi H, Sagawa M, Sato M, et al. A prospective evaluation of transbronchial ultrasonography for assessment of depth of invasion in early bronchogenic squamous cell carcinoma. Lung Cancer 2003;42:43-9. [Crossref] [PubMed]
- Shibuya K, Hoshino H, Chiyo M, et al. Subepithelial vascular patterns in bronchial dysplasias using a high magnification bronchovideoscope. Thorax 2002;57:902-7. [Crossref] [PubMed]
- Shah PL, Kemp SV, Newton RC, et al. Clinical Correlation between Real-Time Endocytoscopy, Confocal Endomicroscopy, and Histopathology in the Central Airways. Respiration 2017;93:51-7. [Crossref] [PubMed]
- Rkein AM, Ozog DM. Photodynamic therapy. Dermatol Clin 2014;32:415-25. x. [Crossref] [PubMed]
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250-81. [Crossref] [PubMed]
- Simone CB 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis 2012;4:63-75. [PubMed]
- Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J 2003;22:535-41. [Crossref] [PubMed]
- Hayata Y, Kato H, Konaka C, et al. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest 1982;81:269-77. [Crossref] [PubMed]
- Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7. [Crossref] [PubMed]
- Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med 2006;38:371-5. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Hagiwara M, et al. Endoscopic evaluation of centrally located early squamous cell carcinoma of the lung. Cancer 2001;91:1142-7. [Crossref] [PubMed]
- Konaka C, Hirano T, Kato H, et al. Comparison of endoscopic features of early-stage squamous cell lung cancer and histological findings. Br J Cancer 1999;80:1435-9. [Crossref] [PubMed]
- Dhillon SS, Demmy TL, Yendamuri S, et al. A Phase I Study of Light Dose for Photodynamic Therapy Using 2-[1-Hexyloxyethyl]-2 Devinyl Pyropheophorbide-a for the Treatment of Non-Small Cell Carcinoma In Situ or Non-Small Cell Microinvasive Bronchogenic Carcinoma: A Dose Ranging Study. J Thorac Oncol 2016;11:234-41. [Crossref] [PubMed]
- Bellnier DA, Greco WR, Nava H, et al. Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother Pharmacol 2006;57:40-5. [Crossref] [PubMed]
- Kato H, Furukawa K, Sato M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003;42:103-11. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Endoscopic treatment of early-stage lung cancer. Cancer Control 2000;7:35-44. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53. [Crossref] [PubMed]
- Kawamura H, Ebara T, Katoh H, et al. Long-term results of curative intraluminal high dose rate brachytherapy for endobronchial carcinoma. Radiat Oncol 2012;7:112. [Crossref] [PubMed]
- Hennequin C, Bleichner O, Tredaniel J, et al. Long-term results of endobronchial brachytherapy: A curative treatment? Int J Radiat Oncol Biol Phys 2007;67:425-30. [Crossref] [PubMed]
- Trédaniel J, Hennequin C, Zalcman G, et al. Prolonged survival after high-dose rate endobronchial radiation for malignant airway obstruction. Chest 1994;105:767-72. [Crossref] [PubMed]
- Pérol M, Caliandro R, Pommier P, et al. Curative irradiation of limited endobronchial carcinomas with high-dose rate brachytherapy. Results of a pilot study. Chest 1997;111:1417-23. [Crossref] [PubMed]
- Aumont-le Guilcher M, Prevost B, Sunyach MP, et al. High-dose-rate brachytherapy for non-small-cell lung carcinoma: a retrospective study of 226 patients. Int J Radiat Oncol Biol Phys 2011;79:1112-6. [Crossref] [PubMed]
- Nomoto Y, Ii N, Murashima S, et al. Endobronchial brachytherapy with curative intent: the impact of reference points setting according to the bronchial diameter. J Radiat Res 2017;58:849-53. [Crossref] [PubMed]
- Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- van Boxem TJ, Venmans BJ, Schramel FM, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Khemasuwan D, Mehta AC, Wang KP. Past, present, and future of endobronchial laser photoresection. J Thorac Dis 2015;7:S380-8. [PubMed]
- Furukawa K, Kato H, Konaka C, et al. Locally recurrent central-type early stage lung cancer <1.0 cm in diameter after complete remission by photodynamic therapy. Chest 2005;128:3269-75. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Toninelli C. Curative Bronchoscopic Laser Therapy for Surgically Resectable Tracheobronchial Tumors: Personal Experience. J Bronchology Interv Pulmonol 2002;9:90-5.
- Mathur PN, Edell E, Sutedja T, et al. Treatment of early stage non-small cell lung cancer. Chest 2003;123:176-80S. [Crossref] [PubMed]
- Infeld M, Gerblich A, Subramanyan S, et al. Focus of bronchial carcinoma in situ eradicated by endobronchial biopsy. Chest 1988;94:1107-9. [Crossref] [PubMed]
- Snyder RW, Mishel HS, Christensen GC 3rd. Bronchogenic carcinoma in situ on the carina eradicated by endobronchial biopsy. Chest 1990;98:1516-7. [Crossref] [PubMed]


