
Pregnancy and Wilson disease: management and outcome of mother and newborns—experiences of a perinatal centre
Introduction
Wilson disease was first identified by Kinnear Wilson in 1912. The incidence of this autosomal-recessive inherited disease of human copper metabolism is reported about 1/30,000 (1,2). Mutation is localized on chromosome 13q14 which encodes an intracellular copper-transporting P-type adenosine triphosphatase (ATP7B) (3). Defective synthesis of ceruloplasmin and impaired copper excretion into bile result in copper accumulation in liver, brain and other tissues (4-6). Liver cirrhosis and nervous system manifestations such as movement disorders and ataxia may result.
Clinical symptoms can vary. Asymptomatic patients are known, but also manifestations with acute liver failure or chronic liver disease, neuropsychiatric disease, or combination of both have been seen (6,7). Furthermore in female patients amenorrhea or oligomenorrhea might be among the first symptoms leading to diagnosis. Often these symptoms are common findings in female patients with Wilson disease. Unknown or untreated Wilson disease usually causes subfertility (8). Conception can be impaired and in cases of pregnancy, it often results in spontaneous and recurrent miscarriage (8-10).
In the general population the spontaneous abortion rate is about 10–20% (11). In the previously published study (136 patients with Wilson disease with 282 pregnancies) of Pfeifferberger et al. (12), 26% of the pregnancies resulted in miscarriage. With respect to the initial maternal clinical manifestation they found the lowest rate in asymptomatic (18%) and in hepatic (20%) manifestation. In contrast they reported the highest rate in patient with neurological manifestation (40%) and undiagnosed Wilson disease (41%). When analyzing treatment regimen, Pfeifferberger et al. (12) found the lowest spontaneous abortion rate for zinc (10%) and D-penicillamine (17%).
Presentation of Wilson disease is more common at an age in the early twenties. At this time patients with Wilson disease are within the reproductive age. As a consequence, in female patients pregnancy becomes a relevant issue for health care professionals since it will not only affect the pregnant women with Wilson disease but also the foetus and off-springs. Prior to introduction of proper medical treatment, successful pregnancies have been rare due to reduced fertility (1,8-10).
However, improvement in treatment of patients with Wilson disease has resulted in successful pregnancy outcomes. Treatment options include the use of the chelating agents D-penicillamine and trientine. Both increase urinary copper excretion. Another therapeutic option is the usage of zinc salts. They affect intestinal copper adsorption and have been shown to be very effective in treating this disorder (5,7).
With appropriate therapy patients can become clinically asymptomatic, in particular when patients are diagnosed in an asymptomatic stage of the disease (4) or when therapy begins early. Furthermore, if early diagnosis is made and proper therapy is initiated, women have a good chance to become pregnant (12-16).
Currently there are three drugs (D-penicillamine, zinc and trientine) used as anticopper agents in patients with Wilson’s disease. They all are effective, but their use during pregnancy and breastfeeding is controversially discussed due to the potential risk of teratogenicity (12,17).
For D-penicillamine an association with severe teratogenicity has been reported in animal models (18) as well as in human case reports (17). Connective tissue defects seem to be associated with the use of D-penicillamine during pregnancy (17). Furthermore functional copper deficiency as a risk factor for birth defects is suggested (12). Trientine theoretically has also teratogenic potential. To date no reports on the teratogenicity of trientine in human have been published, however in animal studies the substance induced fetal cerebral damage (18). Furthermore Dathe et al. (15) reported three uneventful pregnancies under trientine. Zinc seems to have a better safety profile (12). However, to our knowledge, two congenital abnormalities were reported (19).
In summary, therapeutic evolution in treatment of patients with Wilson disease resulted in an increasing number of successful pregnancy outcomes. However, the use of anticopper agents during pregnancy and breastfeeding is still a matter of debate because of possible teratogenicity.
Methods
Twenty-one female patients with Wilson disease treated at the Medical Faculty of the Technical University of Dresden from 1964–2018 were included in this retrospective study. Wilson’s disease was diagnosed according to the scoring system provided by the 8th International Meeting on Wilson disease and Menkes disease and EASL clinical practice guidelines: Wilson disease (20). Nearly 50% of pregnancies were seen before introduction of this guideline and were also included in the present study.
Patient’s chart were reviewed retrospectively for the following criteria: clinical manifestation of Wilson disease, medical treatment prior and during pregnancy, furthermore charts of obstetric department and department of neonatology of our perinatal centre were reviewed for maternal and neonatal course of pregnancy and outcome of mother and newborn in cooperation with obstetrician and paediatrician.
Results
Pregnant women
A total of 32 pregnancies in 22 patients were recorded. The initial clinical manifestations of Wilson’s disease were asymptomatic in 8 patients, mixed in 5 women, hepatic in 5 and neurological in 4 patients. One pregnant patient with hepatic manifestation decided the stop her anticopper medications while pregnant with fatal outcome for both, mother and foetus died. For details see Table 1.
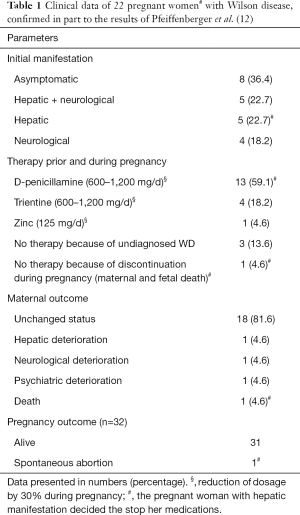
Full table
After having prenatal ultrasound available since 1991 a first ultrasound scan was performed in all women at 20 weeks of gestation for congenital anomalies. All examinations did not show any congenital anomaly. A second scan performed between 32 to 35 weeks of gestation showed growth retardation in 15 out of 32 pregnancies with normal amniotic fluid index.
In 8 of 32 pregnancies oral glucose tolerance test was performed and was positive. In all 8 patients with the diagnosis of gestational diabetes mellitus blood sugar levels remained well controlled during pregnancy through dietary measures.
The mode of delivery varied according to the symptoms of the pregnant women. In asymptomatic women with good physical status vaginal delivery was the first choice, while caesarean section was preferred in case of emergencies or if pathologies were present. The majority of patients of this study did not show any deterioration of symptoms of the disease at controls prior to and during pregnancy. All antenatal laboratory tests were within the normal limits under treatment. Therefore vaginal delivery was possible in 26 of our pregnancies without postpartum complications. Only 5 babies were born by caesarean section.
Newborns
Gestational age of our infants ranged from 34+4 to 40+4 weeks. Postnatal adaptation was uneventful in the majority of our infants. Apgar-Score at 1 minute ranged from 6 to 8, at 5 minutes from 7 to 10 and at 10 minutes from 8 to 10. Umbilical cord arterial pH ranged from 7.09 to 7.41. Birth weight ranged from 2,580 to 3,980 g, length at birth from 46 to 52 cm and head circumference from 31 to 35 cm (for details see Table 2). In 15 infant’s ultrasound scans of the brain, the liver, the kidneys and echocardiography were performed and did not reveal any congenital anomaly.
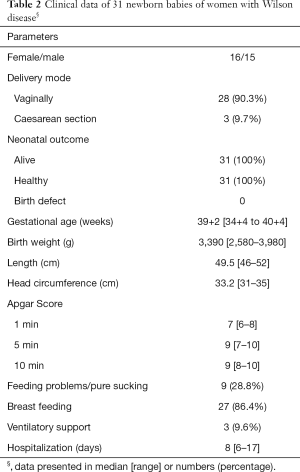
Full table
Respiratory support was required in 3 preterm infants. These infants showed respiratory distress due to wet lung after caesarean section (see case 1–3). Respiratory support with non-invasive ventilation [continuous positive airway pressure (CPAP)] was initiated immediately after birth and symptoms improved within the next two days.
Case 1
A 35-year-old primigravida. Wilson disease was diagnosed at the age of 23 years. Treatment during pregnancy with D-penicillamine was reduced from 1,200 to 900 mg per day. First ultrasound scan revealed no congenital anomalies at 20 weeks of gestation. Second scan at 31 weeks showed growth retardation. Increased maternal blood sugar level of 10.8 mmol/L was found. Oral glucose tolerance test was performed and revealed the diagnosis of gestational diabetes mellitus. Blood sugar levels were normal during pregnancy through dietary measures. The infant was born spontaneously at 36+5 weeks of gestation. A healthy girl weighing 3,680 g with good Apgar score was seen. Initial blood glucose level of the newborn baby was 1.7 mmol/L (normal >2.5 mmol/L) Therefore intravenous glucose was started for two days until oral feeding was completed. Blood glucose levels remained normal in controls. Mother and newborn were discharged home on the 12th postnatal day.
Case 2
A 26-year-old primigravida. Wilson’s disease was diagnosed at the age of nineteen years when she was having symptoms of depression. Treatment during pregnancy was performed with D-penicillamine was reduced from 1,200 to 900 mg per day. First ultrasound scan showed no congenital anomalies at 20 weeks of gestation. The next scan at 31 weeks showed growth retardation. She was admitted because of premature rupture of membranes at 35 weeks of gestation. A preterm infant (male, birth weight 2,950 g) was born at 36 weeks of gestation and showed mild respiratory distress due to wet lung. Respiratory support with non-invasive ventilation (CPAP) was initiated immediately after birth and symptoms improved within the next 24 hours. Despite feeding problems for five days due to poor sucking the postnatal course was unremarkable. Mother and newborn were discharged home on the 17th postnatal day.
Case 3
A 34-year-old primigravida: Wilson disease was diagnosed at the age of 23 years. Treatment during pregnancy was performed with D-penicillamine was reduced from 1,200 to 900 mg per day. The patient developed between 34–36 weeks of gestation preeclampsia with elevated blood pressure showed proteinuria and was hospitalized at our obstetric clinic. Blood pressure was found to be raised (165/110 mmHg), treatment with methyldopa 500 mg was started immediately. Blood pressure remained high between 140/90 and 150/100 mmHg. Caesarean section was performed at 35+3 weeks of gestation due to increasing symptoms despite treatment within 3 to 5 days after hospitalization. The preterm infant (birth weight 2,580 g) needed supplementary oxygen for 6 hours after birth. Despite feeding problems the course of the infant was unremarkable. After caesarean section blood pressure stabilized within a few days and also proteinuria normalized. Mother and newborn were discharged home on the 15th postnatal day.
Prematurity, gestational diabetes mellitus and caesarean section are well known risk factors of mild or moderate respiratory distress in the neonatal period. Breast milk feeding was allowed in all patients and was successful in nearly 80% of nursing mothers over a period up to three month. With the exception of an icterus prolongatus in 4 infants no adverse effects of breast milk feeding were noted. Feeding problems were seen in 9 infants due to poor sucking. Therefore intravenous glucose was started until oral feeding was possible. None of our infants showed major malformations. For details see Table 2.
Discussion
Liver is the primary storage organ of copper. From here copper is distributed in circulation to other tissues such as nervous system, kidneys and eyes. Excessive accumulation of copper due to decreased excretion can adversely affect the liver resulting in hepatic injury or cirrhosis, neurological symptoms such as bradykinesia, ataxia and tremor; Kayser-Fleischer ring and renal tubular damage (6). Untreated Wilson disease results in significant morbidity and can be potentially fatal.
In females at a reproductive age menstrual irregularities due to hepatotoxicity may occur. Furthermore potentially due to copper deposition in the uterus early pregnancy complications such as miscarriages are seen (8,12). The mechanism has been suggested to be similar to that of copper containing contraceptive devices. The contraceptive effect results from deposition of copper in the endometrium (14).
Therapeutic improvements in the past decades have not only improved symptoms and quality of life of patients with Wilson disease. It has also resulted in an increasing number of pregnancies. Therefore the use of anticopper agents become a matter of debate during pregnancy, fetal and maternal outcome, mode of delivery, risk for congenital anomalies and lactation. Mode of delivery varies according to symptoms and the physical status of the pregnant women. In healthy and asymptomatic women vaginal delivery is the first choice. Caesarean section should be reserved for pathologies and emergencies (1).
In our case series, pregnancies in patients with Wilson disease were associated with pregnancy induced complications such as prematurity, hypertension, preeclampsia, and gestational diabetes mellitus (1,14) It may be hypothesized; that pregnant women with Wilson disease are at risk of complicated pregnancy courses with hypertension or preeclampsia (1,3,21). However, it is still a matter of discussion whether this complication is related to the presence of Wilson disease or to the therapy used during pregnancy or not related. Furthermore patients with Wilson disease are also at risk for other complications, such as thrombocytopenia, bleeding, deranged coagulation and placental abruption (3,13,21).
In generally, gestational diabetes mellitus becomes an increasing problem for obstetrics and paediatricians. Therefore oral glucose tolerance test should be performed in all pregnant women with Wilson disease to rule out gestational diabetes mellitus.
Conclusions
Pregnancy in patients with Wilson disease becomes a relevant issue for health care professionals since it will not only affect the pregnant women but also the foetus and off-springs. Chelating therapy in pregnant women with Wilson disease must be continued. However controlled dose reduction down to 60–70 percent of daily dosage should be considered during pregnancy. With proper medical treatment, good compliance of the patients and interdisciplinary monitoring of pregnancy, a successful outcome for mother and newborn can be expected. We did not see major birth defects in our case series. Teratogenic risk seems to be very low after well controlled maternal therapy. Therefore we also recommend breast feeding under maternal chelator exposure. A multi-disciplinary treatment in a high level centre is needed including good prenatal care of pregnant women with Wilson disease, perinatal care provided by specialized obstetricians and neonatologists and appropriate treatment by an expert in Wilson disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the local ethics committee of the Technical University of Dresden (Ethics approval from 20.10.2015, EK 408092015), and written informed consent was obtained from all patients.
References
- Theodiridis TD, Zepiridis L, Athanatos D, et al. Placenta abruption in a women with Wilson’s disease: a case report. Cases J 2009;2:8699. [Crossref] [PubMed]
- Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14:103-13. [Crossref] [PubMed]
- Lee HJ, Seong WJ, Hong SY, et al. Successful pregnancy outcome in a Korean patient with symptomatic Wilson’s disease. Obstet Gynecol Sci 2015;58:409-13. [Crossref] [PubMed]
- Ferenci P. Pathophysiology and clinical features of Wilson disease. Metab Brain Dis 2004;19:229-39. [Crossref] [PubMed]
- Weiss KH, Stremmel W. Evolving perspectives in Wilson disease: diagnosis, treatment and monitoring. Curr Gastroenterol Rep 2012;14:1-7. [Crossref] [PubMed]
- Rosencrantz R, Schilsky M. Wilson disease: pathogenesis and clinical considerations in diagnosis and treatment. Semin Liver Dis 2011;31:245-59. [Crossref] [PubMed]
- Weiss KH, Stremmel W. Clinical considerations for an effective medical therapy in Wilson disease. Ann N Y Acad Sci 2014;1315:81-5. [Crossref] [PubMed]
- Klee JG. Undiagnosed Wilson’s disease as cause of unexplained miscarriage. Lancet 1979;2:423. [Crossref] [PubMed]
- Tarnacka B, Rodo M, Cichy S, et al. Procreation ability in Wilson’s disease. Acta Neurol Scand 2000;101:395-8. [Crossref] [PubMed]
- Sinha S, Taly AB, Prashanth LK, et al. Successful pregnancies and abortions in symptomatic and asymptomatic Wilson’s disease. J Neurol Sci 2004;217:37-40. [Crossref] [PubMed]
- Griebel CP, Halvorsen J, Golemon TB, et al. Management of spontaneous abortion. Am Fam Physician 2005;72:1243-50. [PubMed]
- Pfeiffenberger J, Beinhardt S, Gotthardt DN, et al. Pregnancy in Wilson’s disease: Management and Outcome. Hepatology 2018;67:1261-9. [Crossref] [PubMed]
- Messner U, Guenther HH, Niesert S. Wilson disease and pregnancy. Review of the literature and case report. Z Geburtshilfe Neonatol 1998;202:77-9. [PubMed]
- Malik A, Khawaja A, Sheikh L. Wilson disease in pregnancy: case series and review of literature. BMC Res Notes 2013;6:421. [Crossref] [PubMed]
- Dathe K, Beck E, Schaefer C. Pregnancy outcome after chelation therapy in Wilson disease. Evaluation of the German Embryotox Database. Reprod Toxicol 2016;65:39-45. [Crossref] [PubMed]
- Furman B, Bashiri A, Wiznitzer A, et al. Wilson disease in pregnancy: five successful consecutive pregnancies of the same women. Eur J Obstet Gynecol Reprod Biol 2001;96:232-4. [Crossref] [PubMed]
- Mjolnerod OK, Dommerud SA, Rasmussen K, et al. Congenital connective-tissue defect probably due to D-penicillamine treatment in pregnancy. Lancet 1971;1:673-5. [Crossref] [PubMed]
- Tanaka H, Inomata K, Arima M. Teratogenic effects of triethylene tetramine dihydrochloride on the mouse brain. J Nutr Sci Vitaminol (Tokyo) 1993;39:177-88. [Crossref] [PubMed]
- Brewer GJ, Johnson VD, Dick RD, et al. Treatment of Wilson’s disease with zinc. XVII: treatment during pregnancy. Hepatology 2000;31:364-70. [Crossref] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Członkowska A, Gromadzka G, Büttner J, et al. Clinical features of haemolysis, elevated liver enzymes, and low platelet count syndrome in undiagnosed Wilson disease: report of two case. Arch Gynecol Obstet 2010;281:129-34. [Crossref] [PubMed]


