
Aggressive mycobacterium abscessus on repeated exogenous lipoid pneumonia in the right middle lobe
Introduction
Non-tuberculous mycobacterial (NTM) infection has rarely occurred in the immunocompromised patient. We report NTM infection, superimposed on exogenous lipoid pneumonia, misinterpreted as lung cancer with chest wall spread.
Case presentation
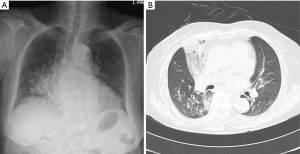
A 65-year-old female, who was never smoker, presented with angina. Her initial chest radiograph (Figure 1A), taken on 8th September, 2003 showed bilateral, parahilar and right lower lobar opacities, suggestive of multifocal acute pneumonia. Chest CT (Figure 1B) showed consolidations, ground glass opacities(GGO) and interlobular septal thickenings in right middle lobe (RML) and both lower lobes. Enhanced CT (Figure 1C) suggested fatty components due to internal negative attenuation in RML consolidation. Afterwards, the history of the patient was carefully examined, and it was remembered that the squalene spray was applied to the nose by herself. The patient was performed bronchoscopy and broncho-alveolar lavage (BAL) 3 times. At the time, exogenous lipoid pneumonia was turned out on pathologic study and symptoms improved after treatment. Chest radiography also showed improvement of RML lesion (Figure 2).

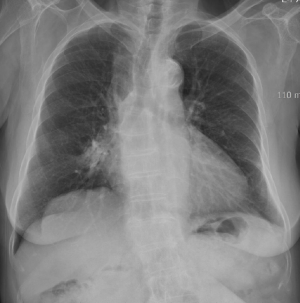
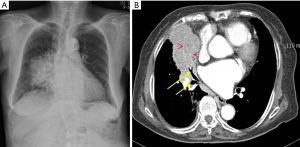
After 12 years, she admitted to the hospital again and took a chest radiography for chest discomfort. Follow-up chest radiograph (Figure 3A) and CT (Figure 3B) showed recurrently ill defined, confluent consolidation in the same area. About 6months later, follow up (FU) chest radiography (Figure 4A) and chest CT (Figure 4B) showed a large mass density in right middle lobe. We interpreted as the primary lung malignancy, developed from exogenous lipoid pneumonia. A sputum study performed at that time showed a negative acid-fast bacilli (AFB) and a positive NTM. CT guided percutaneous needle biopsy (PCNB) was done for RML mass. The pathology findings were consistent with NTM infection including chronic granulomatous inflammation with multinucleated giant cells and focal necrosis.
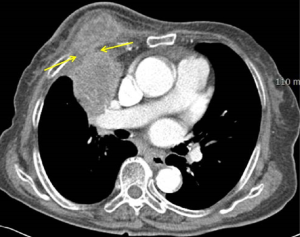
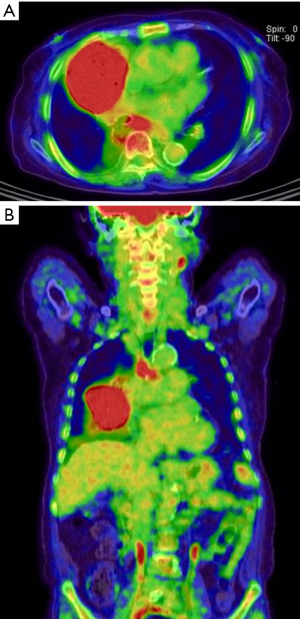
Although the patient took the NTM medication (Rifampicin, Clarithromycin, Ethambutol, Lafutidine, N-acetylcysteine) during about 2 months, chest CT (Figure 5) showed new chest wall involvement of RML mass (arrows). PET-CT showed strong uptake of the RML mass, suggestive of malignant tumor (Figure 6). The operation(“Radicular curettage of thoracic cold abscess”) was done to evaluate the coexisting lung cancer. The pathology revealed NTM (M. abscessus) infection without atypical cell. NTM medication was continued and serial follow up chest radiographs showed decreased size of the RML lesion until 11st, January, 2019.
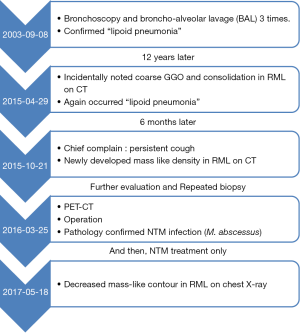
We summarized the patient’s clinical history at a glance (Figure 7). The IRB number is HC19ZESI0015.
Discussion
The diagnosis of pulmonary NTM infection is very difficult to isolate the organism from sputum or bronchoalveolar lavage fluid to present airway colonization (1-3). It is not easy to diagnose as well as false possibility in cultivation process. Among the nontuberculous mycobacterial isolates, respiratory secretion is mainly used. Of these, 61% are Mycobacterium avium-intracellulare complex (MAC), 24% are M. kansasii, 5% are Mycobacterium fortuitum and 10% are other nontuberculous mycobacteria (4).
In addition, NTM is divided into two types, the slow-growing and rapidly growing mycobacterial species, depending on the rate of growth (4,5). The slow-growing species are Mycobacterium avium-intracellulare complex, Mycobacterium kansasii and so on. On the other hand, NTM lung disease caused by a rapidly growing mycobacterial species is not uncommon, but occurs when 80% of it is due to Mycobacterium abscessus (4,5).
M. abscessus has been known to be a common bacterial infection in skin and subcutaneous adipose tissues mainly in postoperative wound infections or systemic infection after organ transplantation in patients with cystic fibrosis (6,7). According to Griffith, M. abscesses were more common in patients with underlying chronic lung disease (70%), and were previously treated with coexistent mycobacterial disease or other specific underlying diseases such as achalasia (8).
Until now, typical CT findings of lung disease cause by mycobacterial abscessus have been known as 90% of branching nodular opacities such as tree-in-bud pattern and bronchiectasis (9 of 10) (9). Although cavitary nodule, lobular consolidation, thin-walled cavity, and peribronchial consolidation were also seen in M. abscessus infection, they were present in less than 30% of patients and occurred less frequently than in MAC infection (10). However, there has been no reports about imaging findings of mass formation, especially with chest wall destruction in M. abscessus infection.
There are little reports about NTM infection, superimposed on underlying disease such as exogenous lipoid pneumonia, but common on bronchiectasis. NTM infection has been reported in patients with exogenous lipoid pneumonia in infants who were medically used oil (11). There has been one case reported in adults, where he was exposed to cooking hume and had lipoid pneumonia due to aspiration of high concentrations of fat aerosols (12). It is presumed that oil probably hinders macrophage and phagocyte function and helps to activate mycobacterium (13). In addition, Kudoh et al., demonstrated that there was increased virulence of NTM during oil inoculation compared with aqueous solutions (14).
Imaging findings of exogenous lipoid pneumonia generally appearing as both lower and middle lobar consolidations, and well-defined ground glass attenuation with interstitial thickening (crazy-paving pattern) is widely known as the most typical form (15). It can also be seen as an ill-defined mass like lesion. However, mass-like consolidation is accompanied by internal negative attenuation, suggestive of a lipid deposit (16). If exogenous lipoid pneumonia such as a kind of aspiration pneumonia or infection occurs in the right middle lobe, its bronchial anatomical direction can disturb to release the infectious material, and finally results in permanent deformity or recurrence of any kinds of infection. The pathogenesis of the disease in middle lobe syndrome is uncertain (17). The narrow diameter and an acute take-off angle create poor condition of drainage and the deep fissures of both the RML and lingula provide barriers to collateral ventilation (18,19).
So, the relation between exogenous lipoid pneumonia and NTM infection is not complicated to suppose. However, the combination of only middle lobe involvement of these two conditions and furthermore mass formation of the necrotic tendency by mycobacterium abscessus is very rare and not reported yet. It looked like lung cancer invading the regional chest wall. We assume that the repetitive exogenous lipoid pneumonia was incompletely treated so that chronic infection such as NTM was superimposed, at some point, rapidly evolving to mimicking necrotic malignancy.
Chest wall abscess related other diseases include tuberculosis, aspergillosis, actinomycosis, and nocardiosis, even though those are rarely seen in more severe immunosuppression state or trauma (including surgery). For example, an extension of pulmonary TB or fungal infection or pleural empyema is so-called, “empyema necessitatis” (20).
We report about the rare condition as extrapleural extending mass by M. abscessus in the right middle lobe, which was solitary, longstanding exogenous lipoid pneumonia site.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis 1979;119:107-59. [PubMed]
- Erasmus JJ, McAdams HP, Farrell MA, et al. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics 1999;19:1487-505. [Crossref] [PubMed]
- Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis 2001;33:1363-74. [Crossref] [PubMed]
- Falkinham JO 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 2011;17:419-24. [Crossref] [PubMed]
- Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections . J Thorac Dis 2014;6:210-20. [PubMed]
- Hazelton TR, Newell JD Jr, Cook JL, et al. CT findings in 14 patients with Mycobacterium chelonae pulmonary infection. AJR Am J Roentgenol 2000;175:413-6. [Crossref] [PubMed]
- Winthrop KL, Mcnelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010;182:977-82. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Han D, Lee KS, Koh WJ, et al. Radiographic and CT findings of nontuberculous mycobacterial pulmonary infection caused by Mycobacterium abscessus. AJR Am J Roentgenol 2003;181:513-7. [Crossref] [PubMed]
- Chung MJ, Lee KS, Koh WJ, et al. Thin-Section CT Findings of Nontuberculous Mycobacterial Pulmonary Diseases: Comparison Between Mycobacterium avium-intracellulare Complex and Mycobacterium abscessus Infection. J Korean Med Sci 2005;20:777-83. [Crossref] [PubMed]
- Marangu D, Pillay K, Banderker E, et al. Exogenous lipoid pneumonia: an important cause of interstitial lung disease in infants. Respirol Case Rep 2018;6:e00356. [Crossref] [PubMed]
- Kobayashi T, Tsuyuguchi K, Yoshida S, et al. A case of Mycobacterium abscessus subsp. massiliense lung disease complicated by lipoid pneumonia. Int J Tuberc Lung Dis 2017;21:124-6. [Crossref] [PubMed]
- Laughlen GF. Studies on pneumonia following naso‐pharyngeal injections of oil. Am J Pathol 1925;1:407-414.1. [PubMed]
- Kudoh S. The virulence of saprophytic acid‐fast bacteria coated with oil or fat, with special reference to an observation on the so‐called atypical acid‐fast bacteria. 1. Experiments with guinea‐pigs on intrapulmonal and subcutaneous inoculation of saprophytic bacteria coated with liquid paraffin. Nihon Saikingaku Zasshi 1962;17:154-61. [Crossref] [PubMed]
- Franquet T, Gimenes A, Border R, et al. Crazy-paving pattern in Exogenous Lipoid Pneumonia: CT-Pathologic Correlation. AJR Am J Roentgenol 1998;170:315-7. [Crossref] [PubMed]
- Giménez A, Franquet T, Prats R, et al. Unusual primary lung tumors: a radiologic-pathologic overview. Radiographics 2002;22:601-19. [Crossref] [PubMed]
- Kwon KY, Myers JL, Swensen SJ, et al. Middle lobe syndrome: A clinicopathological study of 21 patients. Hum Pathol 1995;26:302-7. [Crossref] [PubMed]
- Ayed AK. Resection of the right middle lobe and lingula in children for middle lobe/lingula syndrome. Chest 2004;125:38-42. [Crossref] [PubMed]
- Berrocal T, Madrid C, Novo S, et al. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics 2004;24:e17. [Crossref] [PubMed]
- Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation. RadioGraphics 2003;23:1477-90. [Crossref] [PubMed]


